Cilia in Left-Right Symmetry Breaking
- PMID: 28213464
- PMCID: PMC5629998
- DOI: 10.1101/cshperspect.a028282
Cilia in Left-Right Symmetry Breaking
Abstract
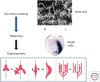
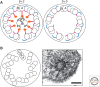
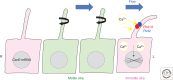
Visceral organs of vertebrates show left-right (L-R) asymmetry with regard to their position and morphology. Cilia play essential role in generating L-R asymmetry. A number of genes required for L-R asymmetry have now been identified in vertebrates, including human, many of which contribute to the formation and motility of cilia. In the mouse embryo, breaking of L-R symmetry occurs in the ventral node, where two types of cilia (motile and immotile) are present. Motile cilia are located at the central region of the node, and generate a leftward fluid flow. These motile cilia at the node are unique in that they rotate in the clockwise direction, unlike other immotile cilia such as airway cilia that show planar beating. The second type of cilia essential for L-R asymmetry is immotile cilia that are peripherally located immotile cilia. They sense a flow-dependent signal, which is either chemical or mechanical in nature. Although Ca2+ signaling is implicated in flow sensing, the precise mechanism remains unknown.
Copyright © 2017 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
-
- Borovina A, Superina S, Voskas D, Ciruna B. 2010. Vangl2 directs the posterior tilting and asymmetric localization of motile primary cilia. Nat Cell Biol 12: 407–412. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
