Central role of T helper 17 cells in chronic hypoxia-induced pulmonary hypertension
- PMID: 28213473
- PMCID: PMC5451600
- DOI: 10.1152/ajplung.00531.2016
Central role of T helper 17 cells in chronic hypoxia-induced pulmonary hypertension
Abstract
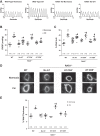
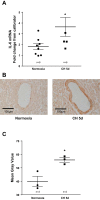
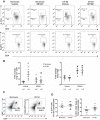
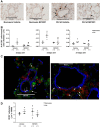
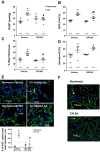
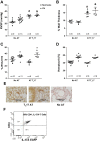
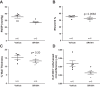
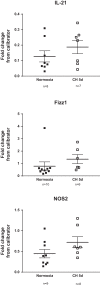
Inflammation is a prominent pathological feature in pulmonary arterial hypertension, as demonstrated by pulmonary vascular infiltration of inflammatory cells, including T and B lymphocytes. However, the contribution of the adaptive immune system is not well characterized in pulmonary hypertension caused by chronic hypoxia. CD4+ T cells are required for initiating and maintaining inflammation, suggesting that these cells could play an important role in the pathogenesis of hypoxic pulmonary hypertension. Our objective was to test the hypothesis that CD4+ T cells, specifically the T helper 17 subset, contribute to chronic hypoxia-induced pulmonary hypertension. We compared indices of pulmonary hypertension resulting from chronic hypoxia (3 wk) in wild-type mice and recombination-activating gene 1 knockout mice (RAG1-/-, lacking mature T and B cells). Separate sets of mice were adoptively transferred with CD4+, CD8+, or T helper 17 cells before normoxic or chronic hypoxic exposure to evaluate the involvement of specific T cell subsets. RAG1-/- mice had diminished right ventricular systolic pressure and arterial remodeling compared with wild-type mice exposed to chronic hypoxia. Adoptive transfer of CD4+ but not CD8+ T cells restored the hypertensive phenotype in RAG1-/- mice. Interestingly, RAG1-/- mice receiving T helper 17 cells displayed evidence of pulmonary hypertension independent of chronic hypoxia. Supporting our hypothesis, depletion of CD4+ cells or treatment with SR1001, an inhibitor of T helper 17 cell development, prevented increased pressure and remodeling responses to chronic hypoxia. We conclude that T helper 17 cells play a key role in the development of chronic hypoxia-induced pulmonary hypertension.
Keywords: CD4 T cells; SR1001; inflammation; interleukin-6; retinoid-related orphan receptor-γτ.
Copyright © 2017 the American Physiological Society.
Figures


References
-
- Ball MK, Waypa GB, Mungai PT, Nielsen JM, Czech L, Dudley VJ, Beussink L, Dettman RW, Berkelhamer SK, Steinhorn RH, Shah SJ, Schumacker PT. Regulation of hypoxia-induced pulmonary hypertension by vascular smooth muscle hypoxia-inducible factor-1α. Am J Respir Crit Care Med 189: 314–324, 2014. doi: 10.1164/rccm.201302-0302OC. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

