Cholesterol up-regulates neuronal G protein-gated inwardly rectifying potassium (GIRK) channel activity in the hippocampus
- PMID: 28213520
- PMCID: PMC5391746
- DOI: 10.1074/jbc.M116.753350
Cholesterol up-regulates neuronal G protein-gated inwardly rectifying potassium (GIRK) channel activity in the hippocampus
Abstract
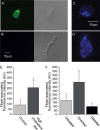
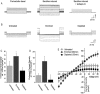
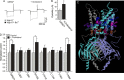
Hypercholesterolemia is a well known risk factor for the development of neurodegenerative disease. However, the underlying mechanisms are mostly unknown. In recent years, it has become increasingly evident that cholesterol-driven effects on physiology and pathophysiology derive from its ability to alter the function of a variety of membrane proteins including ion channels. Yet, the effect of cholesterol on G protein-gated inwardly rectifying potassium (GIRK) channels expressed in the brain is unknown. GIRK channels mediate the actions of inhibitory brain neurotransmitters. As a result, loss of GIRK function can enhance neuron excitability, whereas gain of GIRK function can reduce neuronal activity. Here we show that in rats on a high-cholesterol diet, cholesterol levels in hippocampal neurons are increased. We also demonstrate that cholesterol plays a critical role in modulating neuronal GIRK currents. Specifically, cholesterol enrichment of rat hippocampal neurons resulted in enhanced channel activity. In accordance, elevated currents upon cholesterol enrichment were also observed in Xenopus oocytes expressing GIRK2 channels, the primary GIRK subunit expressed in the brain. Furthermore, using planar lipid bilayers, we show that although cholesterol did not affect the unitary conductance of GIRK2, it significantly enhanced the frequency of channel openings. Last, combining computational and functional approaches, we identified two putative cholesterol-binding sites in the transmembrane domain of GIRK2. These findings establish that cholesterol plays a critical role in modulating GIRK activity in the brain. Because up-regulation of GIRK function can reduce neuronal activity, our findings may lead to novel approaches for prevention and therapy of cholesterol-driven neurodegenerative disease.
Keywords: G protein-gated inwardly rectifying potassium channel; cholesterol; cholesterol-induced channel activation; electrophysiology; hippocampal CA1 pyramidal neurons; lipid bilayer; lipid-protein interaction; molecular docking; neuron; structure-function.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- Dascal N. (1997) Signalling via the G protein-activated K+ channels. Cell Signal. 9, 551–573 - PubMed
-
- Hibino H., Inanobe A., Furutani K., Murakami S., Findlay I., and Kurachi Y. (2010) Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol. Rev. 90, 291–366 - PubMed
-
- Andrade R., Malenka R. C., and Nicoll R. A. (1986) A G protein couples serotonin and GABA(B) receptors to the same channels in hippocampus. Science 234, 1261–1265 - PubMed
-
- Kofuji P., Hofer M., Millen K. J., Millonig J. H., Davidson N., Lester H. A., and Hatten M. E. (1996) Functional analysis of the weaver mutant GIRK2 K+ channel and rescue of weaver granule cells. Neuron 16, 941–952 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

