Identification of Regions in the Spt5 Subunit of DRB Sensitivity-inducing Factor (DSIF) That Are Involved in Promoter-proximal Pausing
- PMID: 28213523
- PMCID: PMC5392697
- DOI: 10.1074/jbc.M116.760751
Identification of Regions in the Spt5 Subunit of DRB Sensitivity-inducing Factor (DSIF) That Are Involved in Promoter-proximal Pausing
Abstract
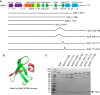
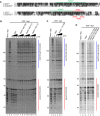
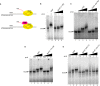
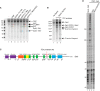
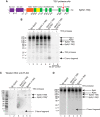
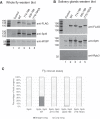
DRB sensitivity-inducing factor (DSIF or Spt4/5) is a conserved transcription elongation factor that both inhibits and stimulates transcription elongation in metazoans. In Drosophila and vertebrates, DSIF together with negative elongation factor (NELF) associates with RNA polymerase II during early elongation and causes RNA polymerase II to pause in the promoter-proximal region of genes. The mechanism of how DSIF establishes pausing is not known. We constructed Spt5 mutant forms of DSIF and tested their capacity to restore promoter-proximal pausing to DSIF-depleted Drosophila nuclear extracts. The C-terminal repeat region of Spt5, which has been implicated in both inhibition and stimulation of elongation, is dispensable for promoter-proximal pausing. A region encompassing KOW4 and KOW5 of Spt5 is essential for pausing, and mutations in KOW5 specifically shift the location of the pause. RNA cross-linking analysis reveals that KOW5 directly contacts the nascent transcript, and deletion of KOW5 disrupts this interaction. Our results suggest that KOW5 is involved in promoter-proximal pausing through contact with the nascent RNA.
Keywords: DSIF; KOW domain; RNA polymerase II; RNA-protein interaction; Spt4/5; mutagenesis; promoter-proximal pausing; transcription elongation factor; transcription regulation.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

