Accurate Quantification of Laminarin in Marine Organic Matter with Enzymes from Marine Microbes
- PMID: 28213541
- PMCID: PMC5394322
- DOI: 10.1128/AEM.03389-16
Accurate Quantification of Laminarin in Marine Organic Matter with Enzymes from Marine Microbes
Abstract
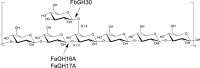
Marine algae produce a variety of glycans, which fulfill diverse biological functions and fuel the carbon and energy demands of heterotrophic microbes. A common approach to analysis of marine organic matter uses acid to hydrolyze the glycans into measurable monosaccharides. The monosaccharides may be derived from different glycans that are built with the same monosaccharides, however, and this approach does not distinguish between glycans in natural samples. Here we use enzymes to digest selectively and thereby quantify laminarin in particulate organic matter. Environmental metaproteome data revealed carbohydrate-active enzymes from marine flavobacteria as tools for selective hydrolysis of the algal β-glucan laminarin. The enzymes digested laminarin into glucose and oligosaccharides, which we measured with standard methods to establish the amounts of laminarin in the samples. We cloned, expressed, purified, and characterized three new glycoside hydrolases (GHs) of Formosa bacteria: two are endo-β-1,3-glucanases, of the GH16 and GH17 families, and the other is a GH30 exo-β-1,6-glucanase. Formosa sp. nov strain Hel1_33_131 GH30 (FbGH30) removed the β-1,6-glucose side chains, and Formosa agariphila GH17A (FaGH17A) and FaGH16A hydrolyzed the β-1,3-glucose backbone of laminarin. Specificity profiling with a library of glucan oligosaccharides and polysaccharides revealed that FaGH17A and FbGH30 were highly specific enzymes, while FaGH16A also hydrolyzed mixed-linked glucans with β-1,4-glucose. Therefore, we chose the more specific FaGH17A and FbGH30 to quantify laminarin in two cultured diatoms, namely, Thalassiosira weissflogii and Thalassiosira pseudonana, and in seawater samples from the North Sea and the Arctic Ocean. Combined, these results demonstrate the potential of enzymes for faster, stereospecific, and sequence-specific analysis of select glycans in marine organic matter.IMPORTANCE Marine algae synthesize substantial amounts of the glucose polymer laminarin for energy and carbon storage. Its concentrations, rates of production by autotrophic organisms, and rates of digestion by heterotrophic organisms remain unknown. Here we present a method based on enzymes that hydrolyze laminarin and enable its quantification even in crude substrate mixtures, without purification. Compared to the commonly used acid hydrolysis, the enzymatic method presented here is faster and stereospecific and selectively cleaves laminarin in mixtures of glycans, releasing only glucose and oligosaccharides, which can be easily quantified with reducing sugar assays.
Keywords: algal bloom; carbon cycle; diatoms; glycobiology; glycoside hydrolase; laminarin; laminarinase; marine microbes; organic matter; β-glucan.
Copyright © 2017 American Society for Microbiology.
Figures

References
-
- Myklestad S. 1974. Production of carbohydrates by marine planktonic diatoms. I. Comparison of nine different species in culture. J Exp Mar Biol Ecol 15:261–274.
-
- Painter TJ. 1983. Algal polysaccharides, p 195–285. In Aspinall GO. (ed), The polysaccharides. Academic Press, New York, NY.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

