A Relay Pathway between Arginine and Tryptophan Metabolism Confers Immunosuppressive Properties on Dendritic Cells
- PMID: 28214225
- PMCID: PMC5337620
- DOI: 10.1016/j.immuni.2017.01.005
A Relay Pathway between Arginine and Tryptophan Metabolism Confers Immunosuppressive Properties on Dendritic Cells
Abstract
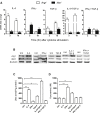
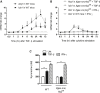
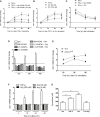
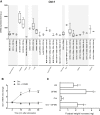
Arginase 1 (Arg1) and indoleamine 2,3-dioxygenase 1 (IDO1) are immunoregulatory enzymes catalyzing the degradation of l-arginine and l-tryptophan, respectively, resulting in local amino acid deprivation. In addition, unlike Arg1, IDO1 is also endowed with non-enzymatic signaling activity in dendritic cells (DCs). Despite considerable knowledge of their individual biology, no integrated functions of Arg1 and IDO1 have been reported yet. We found that IDO1 phosphorylation and consequent activation of IDO1 signaling in DCs was strictly dependent on prior expression of Arg1 and Arg1-dependent production of polyamines. Polyamines, either produced by DCs or released by bystander Arg1+ myeloid-derived suppressor cells, conditioned DCs toward an IDO1-dependent, immunosuppressive phenotype via activation of the Src kinase, which has IDO1-phosphorylating activity. Thus our data indicate that Arg1 and IDO1 are linked by an entwined pathway in immunometabolism and that their joint modulation could represent an important target for effective immunotherapy in several disease settings.
Keywords: Arg1; IDO1; TGF-β; amino acid metabolism; dendritic cell; immune regulation; ornithine; polyamine.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Belladonna M.L., Volpi C., Bianchi R., Vacca C., Orabona C., Pallotta M.T., Boon L., Gizzi S., Fioretti M.C., Grohmann U., Puccetti P. Cutting edge: Autocrine TGF-beta sustains default tolerogenesis by IDO-competent dendritic cells. J. Immunol. 2008;181:5194–5198. - PubMed
-
- Belladonna M.L., Orabona C., Grohmann U., Puccetti P. TGF-beta and kynurenines as the key to infectious tolerance. Trends Mol. Med. 2009;15:41–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

