An enhanced recombinant amino-terminal acetylation system and novel in vivo high-throughput screen for molecules affecting α-synuclein oligomerisation
- PMID: 28214355
- PMCID: PMC5396276
- DOI: 10.1002/1873-3468.12597
An enhanced recombinant amino-terminal acetylation system and novel in vivo high-throughput screen for molecules affecting α-synuclein oligomerisation
Abstract
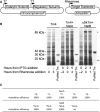
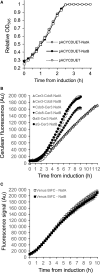
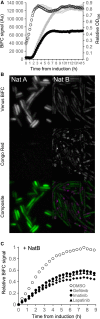
Amino-terminal acetylation is a ubiquitous protein modification affecting the majority of eukaryote proteins to regulate stability and function. We describe an optimised recombinant expression system for rapid production of amino terminal-acetylated proteins within bacteria. We go on to describe the system's use in a fluorescence based in vivo assay for use in the high-throughput screen to identify drugs that impact amino-terminal acetylation-dependent oligomerisation. These new tools and protocols will allow researchers to enhance routine recombinant protein production and identify new molecules for use in research and clinical applications.
Keywords: Nat; Parkinson's disease; Tropomyosin; amino-α-acetyl-transferase; α-synuclein.
© 2017 The Authors. FEBS Letters published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Figures
References
-
- Arnesen T, Van Damme P, Polevoda B, Helsens K, Evjenth R, Colaert N, Varhaug JE, Vandekerckhove J, Lillehaug JR, Sherman F et al (2009) Proteomics analyses reveal the evolutionary conservation and divergence of N‐terminal acetyltransferases from yeast and humans. Proc Natl Acad Sci USA 106, 8157–8162. - PMC - PubMed
-
- Starheim KK, Gevaert K and Arnesen T (2012) Protein N‐terminal acetyltransferases: when the start matters. Trends Biochem Sci 37, 152–161. - PubMed
-
- Gromyko D, Arnesen T, Ryningen A, Varhaug JE and Lillehaug JR (2010) Depletion of the human Nα‐terminal acetyltransferase A induces p53‐dependent apoptosis and p53‐independent growth inhibition. Int J Cancer 127, 2777–2789. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

