Myeloid cells as a target for oligonucleotide therapeutics: turning obstacles into opportunities
- PMID: 28214929
- PMCID: PMC5522630
- DOI: 10.1007/s00262-017-1966-2
Myeloid cells as a target for oligonucleotide therapeutics: turning obstacles into opportunities
Abstract
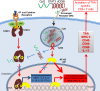
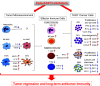
Immunotherapies emerged as an alternative for cancer treatment, yet their clinical efficacies are still limited, especially in case of solid tumors. Myeloid immune cells, such as macrophages and myeloid-derived suppressor cells (MDSCs), are often hijacked by tumors and become pivotal inhibitors of antitumor immunity. Immunosuppressive functions of tumor-associated myeloid cells result from the activity of Signal Transducer and Activator of Transcription 3 (STAT3), a transcription factor with well-defined tumorigenic and tolerogenic roles in human cancers. To overcome challenges in the development of pharmacological STAT3 inhibitors, we recently developed oligonucleotide-based strategies for cell-selective, in vivo STAT3 targeting. Conjugation of a STAT3siRNA or decoy STAT3 inhibitors to synthetic Toll-like Receptor 9 (TLR9) agonists, CpG oligonucleotides, allowed for selective delivery into TLR9-positive cells. Cellular target for CpG-STAT3 inhibitors include non-malignant, tumor-associated myeloid cells, such as polymorphonuclear MDSCs, as well as cancer cells in acute myeloid leukemia, B cell lymphoma and in certain solid tumors. The chemically modified CpG-STAT3 inhibitors resist serum nucleases and thus can be administered intravenously. Their potency relies on the intracellular gain-of-function effect: release of the central immune checkpoint regulator (STAT3) to unleash proinflammatory signaling (CpG/TLR9) in the same antigen-presenting cell. At the cellular level, CpG-STAT3 inhibitors exert two-pronged effect by rescuing T cells from the immune checkpoint control while decreasing survival of cancer cells. In this article, we review the preclinical data on CpG-STAT3 inhibitors and discuss perspectives of using TLR9-targeted delivery of oligonucleotide therapeutics for the generation of novel, more effective and safer cancer immunotherapies.
Keywords: CpG; MDSC; Oligonucleotides; Regulatory myeloid suppressor cells; STAT3; TLR9.
Conflict of interest statement
The authors declare that they have no conflict of interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

