Fibronectin connecting segment-1 peptide inhibits pathogenic leukocyte trafficking and inflammatory demyelination in experimental models of chronic inflammatory demyelinating polyradiculoneuropathy
- PMID: 28215575
- PMCID: PMC5400680
- DOI: 10.1016/j.expneurol.2017.02.012
Fibronectin connecting segment-1 peptide inhibits pathogenic leukocyte trafficking and inflammatory demyelination in experimental models of chronic inflammatory demyelinating polyradiculoneuropathy
Abstract
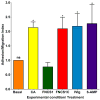
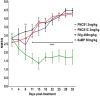
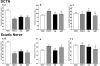
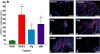
The molecular determinants of pathogenic leukocyte migration across the blood-nerve barrier (BNB) in chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) are unknown. Specific disease modifying therapies for CIDP are also lacking. Fibronectin connecting segment-1 (FNCS1), an alternatively spliced fibronectin variant expressed by microvascular endothelial cells at sites of inflammation in vitro and in situ, is a counterligand for leukocyte α4 integrin (also known as CD49d) implicated in pathogenic leukocyte trafficking in multiple sclerosis and inflammatory bowel disease. We sought to determine the role of FNCS1 in CIDP patient leukocyte trafficking across the BNB in vitro and in severe chronic demyelinating neuritis in vivo using a representative spontaneous murine CIDP model. Peripheral blood mononuclear leukocytes from 7 untreated CIDP patients were independently infused into a cytokine-treated, flow-dependent in vitro BNB model system. Time-lapse digital video microscopy was performed to visualize and quantify leukocyte trafficking, comparing FNCS1 peptide blockade to relevant controls. Fifty 24-week old female B7-2 deficient non-obese diabetic mice with spontaneous autoimmune peripheral polyneuropathy (SAPP) were treated daily with 2mg/kg FNCS1 peptide for 5days via intraperitoneal injection with appropriate controls. Neurobehavioral measures of disease severity, motor nerve electrophysiology assessments and histopathological quantification of inflammation and morphometric assessment of demyelination were performed to determine in vivo efficacy. The biological relevance of FNCS1 and CD49d in CIDP was evaluated by immunohistochemical detection in affected patient sural nerve biopsies. 25μM FNCS1 peptide maximally inhibited CIDP leukocyte trafficking at the human BNB in vitro. FNCS1 peptide treatment resulted in significant improvements in disease severity, motor electrophysiological parameters of demyelination and histological measures of inflammatory demyelination. Microvessels demonstrating FNCS1 expression and CD49d+ leukocytes were seen within the endoneurium of patient nerve biopsies. Taken together, these results imply a role for FNCS1 in pathogenic leukocyte trafficking in CIDP, providing a potential target for therapeutic modulation.
Keywords: Blood-nerve barrier; Chronic inflammatory demyelinating polyradiculoneuropathy; Fibronectin connecting segment-1; Integrin; Leukocyte trafficking; Spontaneous autoimmune peripheral polyneuropathy.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
E.E.U. has received royalties from Baylor Licensing Group for simian virus-40 large T-antigen immortalized human endoneurial endothelial cells and from Springer Science + Business Media for an edited book on laboratory protocols that describes the flow-dependent
Figures



Similar articles
-
The pathogenic relevance of αM-integrin in Guillain-Barré syndrome.Acta Neuropathol. 2016 Nov;132(5):739-752. doi: 10.1007/s00401-016-1599-0. Epub 2016 Jul 26. Acta Neuropathol. 2016. PMID: 27460017 Free PMC article.
-
α(M)β(2)-integrin-intercellular adhesion molecule-1 interactions drive the flow-dependent trafficking of Guillain-Barré syndrome patient derived mononuclear leukocytes at the blood-nerve barrier in vitro.J Cell Physiol. 2012 Dec;227(12):3857-75. doi: 10.1002/jcp.24100. J Cell Physiol. 2012. PMID: 22552879 Free PMC article.
-
Diagnostic value of sural nerve demyelination in chronic inflammatory demyelinating polyneuropathy.Brain. 2001 Dec;124(Pt 12):2427-38. doi: 10.1093/brain/124.12.2427. Brain. 2001. PMID: 11701597
-
Inflammatory neuropathies: pathology, molecular markers and targets for specific therapeutic intervention.Acta Neuropathol. 2015 Oct;130(4):445-68. doi: 10.1007/s00401-015-1466-4. Epub 2015 Aug 12. Acta Neuropathol. 2015. PMID: 26264608 Free PMC article. Review.
-
Chronic Inflammatory Demyelinating Polyneuropathy.Adv Exp Med Biol. 2019;1190:333-343. doi: 10.1007/978-981-32-9636-7_21. Adv Exp Med Biol. 2019. PMID: 31760654 Review.
Cited by
-
Deciphering immune mechanisms in chronic inflammatory demyelinating polyneuropathies.JCI Insight. 2020 Feb 13;5(3):e132411. doi: 10.1172/jci.insight.132411. JCI Insight. 2020. PMID: 32051341 Free PMC article. Review.
-
Biology of the human blood-nerve barrier in health and disease.Exp Neurol. 2020 Jun;328:113272. doi: 10.1016/j.expneurol.2020.113272. Epub 2020 Mar 3. Exp Neurol. 2020. PMID: 32142802 Free PMC article. Review.
-
GDNF enhances human blood-nerve barrier function in vitro via MAPK signaling pathways.Tissue Barriers. 2018;6(4):1-22. doi: 10.1080/21688370.2018.1546537. Epub 2018 Dec 7. Tissue Barriers. 2018. PMID: 30523753 Free PMC article.
-
Differential regulation of tissue-resident and blood-derived macrophages in models of autoimmune and traumatic peripheral nerve injury.Front Immunol. 2024 Nov 19;15:1487788. doi: 10.3389/fimmu.2024.1487788. eCollection 2024. Front Immunol. 2024. PMID: 39628475 Free PMC article.
-
Elimination of activating Fcγ receptors in spontaneous autoimmune peripheral polyneuropathy model protects from neuropathic disease.PLoS One. 2019 Aug 15;14(8):e0220250. doi: 10.1371/journal.pone.0220250. eCollection 2019. PLoS One. 2019. PMID: 31415574 Free PMC article.
References
-
- Boscacci RT, Pfeiffer F, Gollmer K, Sevilla AI, Martin AM, Soriano SF, Natale D, Henrickson S, von Andrian UH, Fukui Y, Mellado M, Deutsch U, Engelhardt B, Stein JV. Comprehensive analysis of lymph node stroma-expressed Ig superfamily members reveals redundant and nonredundant roles for ICAM-1, ICAM-2, and VCAM-1 in lymphocyte homing. Blood. 2010;116:915–925. - PMC - PubMed
-
- Brannagan TH., 3rd Current treatments of chronic immune-mediated demyelinating polyneuropathies. Muscle & nerve. 2009;39:563–578. - PubMed
-
- Chia L, Fernandez A, Lacroix C, Adams D, Plante V, Said G. Contribution of nerve biopsy findings to the diagnosis of disabling neuropathy in the elderly. A retrospective review of 100 consecutive patients. Brain : a journal of neurology. 1996;119(Pt 4):1091–1098. - PubMed
-
- Dalakas MC. Pathogenesis of immune-mediated neuropathies. Biochimica et biophysica acta. 2015;1852:658–666. - PubMed
-
- Dalakas MC, Medscape Advances in the diagnosis, pathogenesis and treatment of CIDP. Nature reviews. Neurology. 2011;7:507–517. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

