Complementopathies
- PMID: 28215731
- PMCID: PMC5513767
- DOI: 10.1016/j.blre.2017.02.003
Complementopathies
Abstract
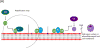
The complement system is an essential part of the innate immune system that requires careful regulation to ensure responses are appropriately directed against harmful pathogens, while preventing collateral damage to normal host cells and tissues. While deficiency in some components of the complement pathway is associated with increased susceptibility to certain infections, it has also become clear that inappropriate activation of complement is an important contributor to human disease. A number of hematologic disorders are driven by complement, and these disorders may be termed "complementopathies". This includes paroxysmal nocturnal hemoglobinuria (PNH), atypical hemolytic uremic syndrome (aHUS), cold agglutinin disease (CAD) and other related disorders, which will be the focus of this review. A better understanding of the central role of the complement system in the pathophysiology of these disorders may allow for application of therapies directed at blocking the complement cascade.
Keywords: Alternative pathway of complement; Atypical hemolytic uremic syndrome; Cold agglutinin disease; Complementopathy; HELLP syndrome; Paroxysmal nocturnal hemoglobinuria.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
R.A.B. serves on the scientific advisory board for Alexion Pharmaceuticals, Achillion Pharmaceuticals and Apellis Pharmaceuticals. A.C.B. has no relevant conflicts of interest.
Figures





References
-
- Kaufmann SH. Immunology’s foundation: the 100-year anniversary of the Nobel Prize to Paul Ehrlich and Elie Metchnikoff. Nat Immunol. 2008;9(7):705–712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

