Successful application of large microneedle patches by human volunteers
- PMID: 28216463
- PMCID: PMC5364775
- DOI: 10.1016/j.ijpharm.2017.02.011
Successful application of large microneedle patches by human volunteers
Abstract
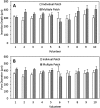
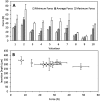
We describe, for the first time, the design, production and evaluation of large microneedle patches. Such systems, based on 16 individual microneedle arrays (needle height 600μm), were prepared from aqueous blends of 15% w/w Gantrez® S97 and 7.5% w/w poly(ethyleneglycol) 10,000Da. Ester-based crosslinking was confirmed by FTIR and mechanical strength was good. Insertion depths in a validated skin model were approximately 500μm. Ten human volunteers successfully self-inserted the microneedles of these larger patches in their skin, following appropriate instruction, as confirmed by transepidermal water loss measurements. The mean insertion depth ranged between 300 and 450μm over the area of the large patches. That this was not significantly different to a single unit MN patch self-applied by the same volunteers is encouraging. Microneedle patch sizes much larger than the 1-2cm2 will be required if this technology is to be successfully translated to clinic for delivery of drug substances. The work described here suggests that use of such larger patches by patients can be successful, potentially opening up the possibility for a significant expansion of the size of the market for transdermal drug delivery.
Keywords: Clinical translation; Large patches; Microneedles; Self-application.
Copyright © 2017 The Author(s). Published by Elsevier B.V. All rights reserved.
Figures






References
-
- Birchall J.C., Clemo R., Anstey A., John D.N. Microneedles in clinical practice–an exploratory study into the opinions of healthcare professionals and the public. Pharm. Res. 2011;28:95–106. - PubMed
-
- Donnelly R.F., Singh T.R.R., Morrow D.I.J., Woolfson A.D. Wiley; 2012. Microneedle-Mediated Transdermal and Intradermal Drug Delivery.
-
- Donnelly R.F., Moffatt K., Alkilani A.Z., Vicente-Pérez E.M., Barry J., McCrudden M.T.C., Woolfson A.D. Hydrogel-forming microneedle arrays can be effectively inserted in skin by self-application: a pilot study centred on pharmacist intervention and a patient information leaflet. Pharm. Res. 2014:1–11. - PubMed
-
- Donnelly R.F., McCrudden M.T.C., Zaid Alkilani A., Larrañeta E., McAlister E., Courtenay A.J., Kearney M., Singh T.R.R., McCarthy H.O., Kett V.L., Caffarel-Salvador E., Al-Zahrani S., Woolfson A.D. Hydrogel-forming microneedles prepared from super swellingñ polymers combined with lyophilised wafers for transdermal drug delivery. PLoS One. 2014;9:e111547. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

