Iron isotopic fractionation between silicate mantle and metallic core at high pressure
- PMID: 28216664
- PMCID: PMC5321738
- DOI: 10.1038/ncomms14377
Iron isotopic fractionation between silicate mantle and metallic core at high pressure
Abstract
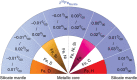
The +0.1‰ elevated 56Fe/54Fe ratio of terrestrial basalts relative to chondrites was proposed to be a fingerprint of core-mantle segregation. However, the extent of iron isotopic fractionation between molten metal and silicate under high pressure-temperature conditions is poorly known. Here we show that iron forms chemical bonds of similar strengths in basaltic glasses and iron-rich alloys, even at high pressure. From the measured mean force constants of iron bonds, we calculate an equilibrium iron isotope fractionation between silicate and iron under core formation conditions in Earth of ∼0-0.02‰, which is small relative to the +0.1‰ shift of terrestrial basalts. This result is unaffected by small amounts of nickel and candidate core-forming light elements, as the isotopic shifts associated with such alloying are small. This study suggests that the variability in iron isotopic composition in planetary objects cannot be due to core formation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

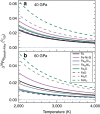
 values for Fe92Ni8, Fe85Si15 and Fe3S evaluated based on the NRIXS data from Lin et al. using the SciPhon software; open square and diamonds: bridgmanite and Fe reported by Shahar et al.; dashed line: bridgmanite reported by Rustad and Yin. The error bars are 95% confidence intervals. Each high-pressure data point was measured at least 20 times and as many as 47 times. Solid lines: linear fits to the data for the basaltic glass, Fe and iron-rich alloys. Note the large shift in
values for Fe92Ni8, Fe85Si15 and Fe3S evaluated based on the NRIXS data from Lin et al. using the SciPhon software; open square and diamonds: bridgmanite and Fe reported by Shahar et al.; dashed line: bridgmanite reported by Rustad and Yin. The error bars are 95% confidence intervals. Each high-pressure data point was measured at least 20 times and as many as 47 times. Solid lines: linear fits to the data for the basaltic glass, Fe and iron-rich alloys. Note the large shift in  value of the basaltic glass (a) at ∼30 GPa, which corresponds to structural changes in the glass.
value of the basaltic glass (a) at ∼30 GPa, which corresponds to structural changes in the glass.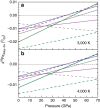
References
-
- Beard B. L. & Johnson C. M. Fe isotope variations in the modern and ancient earth and other planetary bodies. Rev. Mineral. Geochem. 55, 319–357 (2004).
-
- Poitrasson F., Halliday A. N., Lee D.-C., Levasseur S. & Teutsch N. Iron isotope differences between Earth, Moon, Mars and Vesta as possible records of contrasted accretion mechanisms. Earth Planet. Sci. Lett. 223, 253–266 (2004).
-
- Williams H. M. et al.. Iron isotope fractionation and the oxygen fugacity of the mantle. Science 304, 1656–1659 (2004). - PubMed
-
- Weyer S. et al.. Iron isotope fractionation during planetary differentiation. Earth Planet. Sci. Lett. 240, 251–264 (2005).
-
- Schoenberg R. & Blanckenburg F. V. Modes of planetary-scale Fe isotope fractionation. Earth Planet. Sci. Lett. 252, 342–359 (2006).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

