Resting State fMRI in Mice Reveals Anesthesia Specific Signatures of Brain Functional Networks and Their Interactions
- PMID: 28217085
- PMCID: PMC5289996
- DOI: 10.3389/fncir.2017.00005
Resting State fMRI in Mice Reveals Anesthesia Specific Signatures of Brain Functional Networks and Their Interactions
Abstract
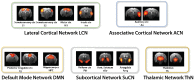
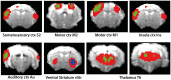
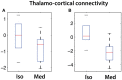
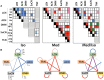
fMRI studies in mice typically require the use of anesthetics. Yet, it is known that anesthesia alters responses to stimuli or functional networks at rest. In this work, we have used Dual Regression analysis Network Modeling to investigate the effects of two commonly used anesthetics, isoflurane and medetomidine, on rs-fMRI derived functional networks, and in particular to what extent anesthesia affected the interaction within and between these networks. Experimental data have been used from a previous study (Grandjean et al., 2014). We applied multivariate ICA analysis and Dual Regression to infer the differences in functional connectivity between isoflurane- and medetomidine-anesthetized mice. Further network analysis was performed to investigate within- and between-network connectivity differences between these anesthetic regimens. The results revealed five major networks in the mouse brain: lateral cortical, associative cortical, default mode, subcortical, and thalamic network. The anesthesia regime had a profound effect both on within- and between-network interactions. Under isoflurane anesthesia predominantly intra- and inter-cortical interactions have been observed, with only minor interactions involving subcortical structures and in particular attenuated cortico-thalamic connectivity. In contrast, medetomidine-anesthetized mice displayed subcortical functional connectivity including interactions between cortical and thalamic ICA components. Combining the two anesthetics at low dose resulted in network interaction that constituted the superposition of the interaction observed for each anesthetic alone. The study demonstrated that network modeling is a promising tool for analyzing the brain functional architecture in mice and comparing alterations therein caused by different physiological or pathological states. Understanding the differential effects of anesthetics on brain networks and their interaction is essential when interpreting fMRI data recorded under specific physiological and pathological conditions.
Keywords: anesthesia; brain network analysis; dual regression; fMRI; isoflurane; medetomidine; network interactions; rodent fMRI.
Figures
Similar articles
-
Optimization of anesthesia protocol for resting-state fMRI in mice based on differential effects of anesthetics on functional connectivity patterns.Neuroimage. 2014 Nov 15;102 Pt 2:838-47. doi: 10.1016/j.neuroimage.2014.08.043. Epub 2014 Aug 28. Neuroimage. 2014. PMID: 25175535
-
Combined resting state-fMRI and calcium recordings show stable brain states for task-induced fMRI in mice under combined ISO/MED anesthesia.Neuroimage. 2021 Dec 15;245:118626. doi: 10.1016/j.neuroimage.2021.118626. Epub 2021 Oct 9. Neuroimage. 2021. PMID: 34637903
-
Increasing isoflurane dose reduces homotopic correlation and functional segregation of brain networks in mice as revealed by resting-state fMRI.Sci Rep. 2018 Jul 12;8(1):10591. doi: 10.1038/s41598-018-28766-3. Sci Rep. 2018. PMID: 30002419 Free PMC article.
-
Mapping cognitive and emotional networks in neurosurgical patients using resting-state functional magnetic resonance imaging.Neurosurg Focus. 2020 Feb 1;48(2):E9. doi: 10.3171/2019.11.FOCUS19773. Neurosurg Focus. 2020. PMID: 32006946 Free PMC article. Review.
-
Brain complex network analysis by means of resting state fMRI and graph analysis: will it be helpful in clinical epilepsy?Epilepsy Behav. 2014 Sep;38:71-80. doi: 10.1016/j.yebeh.2013.11.019. Epub 2013 Dec 27. Epilepsy Behav. 2014. PMID: 24374054 Review.
Cited by
-
A Role for the Claustrum in Salience Processing?Front Neuroanat. 2019 Jun 19;13:64. doi: 10.3389/fnana.2019.00064. eCollection 2019. Front Neuroanat. 2019. PMID: 31275119 Free PMC article.
-
Transient brain activity dynamics discriminate levels of consciousness during anesthesia.Commun Biol. 2024 Jun 10;7(1):716. doi: 10.1038/s42003-024-06335-x. Commun Biol. 2024. PMID: 38858589 Free PMC article.
-
Cranioplastic Surgery and Acclimation Training for Awake Mouse fMRI.Bio Protoc. 2021 Apr 5;11(7):e3972. doi: 10.21769/BioProtoc.3972. eCollection 2021 Apr 5. Bio Protoc. 2021. PMID: 33889666 Free PMC article.
-
Early Stage Alterations in White Matter and Decreased Functional Interhemispheric Hippocampal Connectivity in the 3xTg Mouse Model of Alzheimer's Disease.Front Aging Neurosci. 2019 Mar 22;11:39. doi: 10.3389/fnagi.2019.00039. eCollection 2019. Front Aging Neurosci. 2019. PMID: 30967770 Free PMC article.
-
Resting-state functional MRI reveals altered brain connectivity and its correlation with motor dysfunction in a mouse model of Huntington's disease.Sci Rep. 2017 Dec 1;7(1):16742. doi: 10.1038/s41598-017-17026-5. Sci Rep. 2017. PMID: 29196686 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

