Feedback Activation of Basic Fibroblast Growth Factor Signaling via the Wnt/β-Catenin Pathway in Skin Fibroblasts
- PMID: 28217097
- PMCID: PMC5289949
- DOI: 10.3389/fphar.2017.00032
Feedback Activation of Basic Fibroblast Growth Factor Signaling via the Wnt/β-Catenin Pathway in Skin Fibroblasts
Abstract
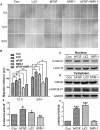
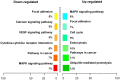
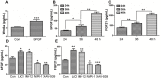
Skin wound healing is a complex process requiring the coordinated behavior of many cell types, especially in the proliferation and migration of fibroblasts. Basic fibroblast growth factor (bFGF) is a member of the FGF family that promotes fibroblast migration, but the underlying molecular mechanism remains elusive. The present RNA sequencing study showed that the expression levels of several canonical Wnt pathway genes, including Wnt2b, Wnt3, Wnt11, T-cell factor 7 (TCF7), and Frizzled 8 (FZD8) were modified by bFGF stimulation in fibroblasts. Enzyme-linked immunosorbent assay (ELISA) analysis also showed that Wnt pathway was activated under bFGF treatment. Furthermore, treatment of fibroblasts with lithium chloride or IWR-1, an inducer and inhibitor of the Wnt signaling pathway, respectively, promoted and inhibited cell migration. Also, levels of cytosolic glycogen synthase kinase 3 beta phosphorylated at serine9 (pGSK3β Ser9) and nuclear β-catenin were increased upon exposure to bFGF. Molecular and biochemical assays indicated that phosphoinositide 3-kinase (PI3K) signaling activated the GSK3β/β-catenin/Wnt signaling pathway via activation of c-Jun N-terminal kinase (JNK), suggesting that PI3K and JNK act at the upstream of β-catenin. In contrast, knock-down of β-catenin delayed fibroblast cell migration even under bFGF stimulation. RNA sequencing analysis of β-catenin knock-down fibroblasts demonstrated that β-catenin positively regulated the transcription of bFGF and FGF21. Moreover, FGF21 treatment activated AKT and JNK, and accelerated fibroblast migration to a similar extent as bFGF does. In addition, ELISA analysis demonstrated that both of bFGF and FGF21 were auto secretion factor and be regulated by Wnt pathway stimulators. Taken together, our analyses define a feedback regulatory loop between bFGF (FGF21) and Wnt signaling acting through β-catenin in skin fibroblasts.
Keywords: GSK3β; Wnt signaling pathway; bFGF; cell migration; transcriptome; β-catenin.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

