Priming Neural Circuits to Modulate Spinal Reflex Excitability
- PMID: 28217104
- PMCID: PMC5289977
- DOI: 10.3389/fneur.2017.00017
Priming Neural Circuits to Modulate Spinal Reflex Excitability
Abstract
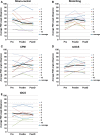
While priming is most often thought of as a strategy for modulating neural excitability to facilitate voluntary motor control, priming stimulation can also be utilized to target spinal reflex excitability. In this application, priming can be used to modulate the involuntary motor output that often follows central nervous system injury. Individuals with spinal cord injury (SCI) often experience spasticity, for which antispasmodic medications are the most common treatment. Physical therapeutic/electroceutic interventions offer an alternative treatment for spasticity, without the deleterious side effects that can accompany pharmacological interventions. While studies of physical therapeutic/electroceutic interventions have been published, a systematic comparison of these approaches has not been performed. The purpose of this study was to compare four non-pharmacological interventions to a sham-control intervention to assess their efficacy for spasticity reduction. Participants were individuals (n = 10) with chronic SCI (≥1 year) who exhibited stretch-induced quadriceps spasticity. Spasticity was quantified using the pendulum test before and at two time points after (immediate, 45 min delayed) each of four different physical therapeutic/electroceutic interventions, plus a sham-control intervention. Interventions included stretching, cyclic passive movement (CPM), transcutaneous spinal cord stimulation (tcSCS), and transcranial direct current stimulation (tDCS). The sham-control intervention consisted of a brief ramp-up and ramp-down of knee and ankle stimulation while reclined with legs extended. The order of interventions was randomized, and each was tested on a separate day with at least 48 h between sessions. Compared to the sham-control intervention, stretching, CPM, and tcSCS were associated with a significantly greater reduction in spasticity immediately after treatment. While the immediate effect was largest for stretching, the reduction persisted for 45 min only for the CPM and tcSCS interventions. tDCS had no immediate or delayed effects on spasticity when compared to sham-control. Interestingly, the sham-control intervention was associated with significant within-session increases in spasticity, indicating that spasticity increases with immobility. These findings suggest that stretching, CPM, and tcSCS are viable non-pharmacological alternatives for reducing spasticity, and that CPM and tcSCS have prolonged effects. Given that the observed effects were from a single-session intervention, future studies should determine the most efficacious dosing and timing strategies.
Keywords: cyclic passive movement; spasticity; spinal cord injury; stretching; tanscranial direct current stimulation; transcutaneous spinal cord stimulation.
Figures


References
-
- Taricco M, Pagliacci MC, Telaro E, Adone R. Pharmacological interventions for spasticity following spinal cord injury: results of a Cochrane systematic review. Eura Medicophys (2006) 42(1):5–15. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

