High Endothelial Venules and Other Blood Vessels: Critical Regulators of Lymphoid Organ Development and Function
- PMID: 28217126
- PMCID: PMC5289948
- DOI: 10.3389/fimmu.2017.00045
High Endothelial Venules and Other Blood Vessels: Critical Regulators of Lymphoid Organ Development and Function
Abstract
The blood vasculature regulates both the development and function of secondary lymphoid organs by providing a portal for entry of hemopoietic cells. During the development of lymphoid organs in the embryo, blood vessels deliver lymphoid tissue inducer cells that initiate and sustain the development of lymphoid tissues. In adults, the blood vessels are structurally distinct from those in other organs due to the requirement for high levels of lymphocyte recruitment under non-inflammatory conditions. In lymph nodes (LNs) and Peyer's patches, high endothelial venules (HEVs) especially adapted for lymphocyte trafficking form a spatially organized network of blood vessels, which controls both the type of lymphocyte and the site of entry into lymphoid tissues. Uniquely, HEVs express vascular addressins that regulate lymphocyte entry into lymphoid organs and are, therefore, critical to the function of lymphoid organs. Recent studies have demonstrated important roles for CD11c+ dendritic cells in the induction, as well as the maintenance, of vascular addressin expression and, therefore, the function of HEVs. Tertiary lymphoid organs (TLOs) are HEV containing LN-like structures that develop inside organized tissues undergoing chronic immune-mediated inflammation. In autoimmune lesions, the development of TLOs is thought to exacerbate disease. In cancerous tissues, the development of HEVs and TLOs is associated with improved patient outcomes in several cancers. Therefore, it is important to understand what drives the development of HEVs and TLOs and how these structures contribute to pathology. In several human diseases and experimental animal models of chronic inflammation, there are some similarities between the development and function of HEVs within LN and TLOs. This review will summarize current knowledge of how hemopoietic cells with lymphoid tissue-inducing, HEV-inducing, and HEV-maintaining properties are recruited from the bloodstream to induce the development and control the function of lymphoid organs.
Keywords: blood vessels; ectopic lymphoid structures; high endothelial venules; lymph nodes; mucosal addressin; peripheral node addressin; tertiary lymphoid organs.
Figures
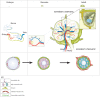
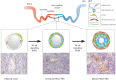
References
-
- Bombardieri M, Barone F, Humby F, Kelly S, McGurk M, Morgan P, et al. Activation-induced cytidine deaminase expression in follicular dendritic cell networks and interfollicular large B cells supports functionality of ectopic lymphoid neogenesis in autoimmune sialoadenitis and MALT lymphoma in Sjogren’s syndrome. J Immunol (2007) 179(7):4929–38.10.4049/jimmunol.179.7.4929 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

