Tumor-Associated Lymphatic Vessels Upregulate PDL1 to Inhibit T-Cell Activation
- PMID: 28217128
- PMCID: PMC5289955
- DOI: 10.3389/fimmu.2017.00066
Tumor-Associated Lymphatic Vessels Upregulate PDL1 to Inhibit T-Cell Activation
Abstract
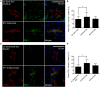
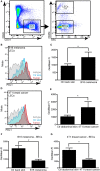
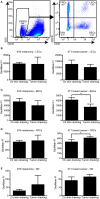
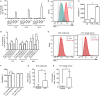
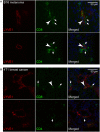
Tumor-associated lymphatic vessels (LVs) play multiple roles during tumor progression, including promotion of metastasis and regulation of antitumor immune responses by delivering antigen from the tumor bed to draining lymph nodes (LNs). Under steady-state conditions, LN resident lymphatic endothelial cells (LECs) have been found to maintain peripheral tolerance by directly inhibiting autoreactive T-cells. Similarly, tumor-associated lymphatic endothelium has been suggested to reduce antitumor T-cell responses, but the mechanisms that mediate this effect have not been clarified. Using two distinct experimental tumor models, we found that tumor-associated LVs gain expression of the T-cell inhibitory molecule PDL1, similar to LN resident LECs, whereas tumor-associated blood vessels downregulate PDL1. The observed lymphatic upregulation of PDL1 was likely due to IFN-g released by stromal cells in the tumor microenvironment. Furthermore, we found that blocking PDL1 results in increased T-cell stimulation by antigen-presenting LECs in vitro. Taken together, our data suggest that peripheral, tumor-associated lymphatic endothelium contributes to T-cell inhibition, by a mechanism similar to peripheral tolerance maintenance described for LN resident LECs. These findings may have clinical implications for cancer therapy, as lymphatic expression of PDL1 could represent a new biomarker to select patients for immunotherapy with PD1 or PDL1 inhibitors.
Keywords: PD1; T-cell exhaustion; abortive proliferation; immune checkpoint; lymph node; peripheral tolerance; tumor vasculature; tumor-induced immunosuppression.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

