α1-antitrypsin Deficiency: A Misfolded Secretory Protein Variant with Unique Effects on the Endoplasmic Reticulum
- PMID: 28217691
- PMCID: PMC5310618
- DOI: 10.1515/ersc-2016-0004
α1-antitrypsin Deficiency: A Misfolded Secretory Protein Variant with Unique Effects on the Endoplasmic Reticulum
Abstract
In the classical form of α1-antitrypsin deficiency (ATD) a point mutation leads to accumulation of a misfolded secretory glycoprotein in the endoplasmic reticulum (ER) of liver cells and so ATD has come to be considered a prototypical ER storage disease. It is associated with two major types of clinical disorders, chronic obstructive pulmonary disease (COPD) by loss-of-function mechanisms and hepatic cirrhosis and carcinogenesis by gain-of-function mechanisms. The lung disease predominantly results from proteolytic damage to the pulmonary connective tissue matrix because of reduced levels of protease inhibitor activity of α1-anitrypsin (AT) in the circulating blood and body fluids. Cigarette smoking is a powerful disease-promoting modifier but other modifiers are known to exist because variation in the lung disease phenotype is still found in smoking and non-smoking homozygotes. The liver disease is highly likely to be caused by the proteotoxic effects of intracellular misfolded protein accumulation and a high degree of variation in the hepatic phenotype among affected homozygotes has been hypothetically attributed to genetic and environmental modifiers that alter proteostasis responses. Liver biopsies of homozygotes show intrahepatocytic inclusions with dilation and expansion of the ER and recent studies of iPS-derived hepatocyte-like cells from individuals with ATD indicate that the changes in the ER directly vary with the hepatic phenotype i.e there is much lesser alteration in the ER in cells derived from homozygotes that do not have clinically significant liver disease. From a signaling perspective, studies in mammalian cell line and animal models expressing the classical α1-antitrypsin Z variant (ATZ) have found that ER signaling is perturbed in a relatively unique way with powerful activation of autophagy and the NFκB pathway but relatively limited, if any, UPR signaling. It is still not known how much these unique structural and functional changes and the variation among affected homozygotes relate to the tendency of this variant to polymerize and aggregate and/or to the repertoire of proteostasis mechanisms that are activated.
Keywords: Autophagy; ER storage; Proteostasis.
Conflict of interest statement
Conflict of interest: Author states no conflict of interest
Figures
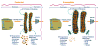

References
-
- Laurell C-B, Eriksson S. The electrophoretic α1-globulin pattern of serum in α1-antitrypsin deficiency. Scand J Clin Lab Invest. 1963;15:132–40. - PubMed
-
- Brantly ML, Paul LD, Miller BH, et al. Clinical features and history of the destructive lung disease associated with alpha-1-antitrypsin deficiency of adults with pulmonary symptoms. Am Rev Respir Dis. 1988;128:327–36. - PubMed
-
- Janus ED, Philips NT, Carrell RW. Smoking, lung function and α1-antitrypsin deficiency. Lancet. 1985;1:152–4. - PubMed
-
- Sharp HL, Bridges RA, Krivit W, Freier EF. Cirrhosis associated with alpha-1-antitrypsin deficiency: a previously unrecognized inherited disorder. J Lab Clin Med. 1969;73:934–939. - PubMed
-
- Eriksson S, Carlson J, Velez R. Risk of cirrhosis and primary liver cancer in alpha1-antitrypsin deficiency. N Engl J Med. 1986;314:736–739. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
