Pharmacokinetics and Pharmacodynamics of Tolvaptan in Autosomal Dominant Polycystic Kidney Disease: Phase 2 Trials for Dose Selection in the Pivotal Phase 3 Trial
- PMID: 28218410
- PMCID: PMC5480307
- DOI: 10.1002/jcph.880
Pharmacokinetics and Pharmacodynamics of Tolvaptan in Autosomal Dominant Polycystic Kidney Disease: Phase 2 Trials for Dose Selection in the Pivotal Phase 3 Trial
Abstract
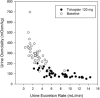
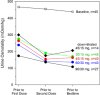
In the pivotal TEMPO 3:4 trial, the arginine vasopressin V2-receptor antagonist tolvaptan reduced the rate of kidney growth in patients with autosomal dominant polycystic kidney disease. Tolvaptan was initiated as daily morning/afternoon doses of 45/15 mg, and uptitrated weekly to 60/30 mg and 90/30 mg according to patient-reported tolerability. The current report describes 3 phase 2 trials in adult autosomal dominant polycystic kidney disease subjects that were the basis for the titrated split-dose regimen: a single ascending-dose trial (tolvaptan 15 to 120 mg; n = 11), a multiple split-dose trial (tolvaptan 15/15 mg, 30/0 mg, 30/15 mg, and 30/30 mg; n = 37), and an 8-week open-label safety and efficacy trial in 46 of the 48 subjects who participated in the prior 2 trials (tolvaptan 30/15 mg, 45/15 mg, 60/30 mg, and 90/30 mg). Urine osmolality (Uosm ) was chosen as the biomarker of V2 receptor inhibition. Two tolvaptan doses per day were necessary to suppress Uosm to <300 mOsm/kg for 24 hours. The 45/15-mg regimen was well tolerated and effective in suppressing Uosm in >50% of subjects. Therefore, this regimen was selected as the starting regimen for the TEMPO 3:4 trial. The 90/30-mg regimen suppressed Uosm in 85% of subjects tested; however, only 28/46 subjects agreed to uptitrate to 90/30 mg due to tolerability. Higher concentrations of tolvaptan were less well tolerated, resulting in adverse events of pollakiuria, thirst, polyuria, nocturia, and a higher number of times out of bed to urinate. Subjects who agreed to uptitrate to 90/30 mg had lower eGFR than those who did not uptitrate.
Keywords: Tolvaptan; autosomal dominant polycystic kidney disease; pharmacodynamics; pharmacokinetics; tolerability; urine osmolality.
© 2017 The Authors. The Journal of Clinical Pharmacology Published by Wiley Periodicals, Inc. on behalf of American College of Clinical Pharmacology.
Figures




References
-
- Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2‐receptor antagonist, for hyponatremia. N Engl J Med. 2006;355(20):2099–2112. - PubMed
-
- Baumgarten R, van de Pol MH, Deen PM, van Os CH, Wetzels JF. Dissociation between urine osmolality and urinary excretion of aquaporin‐2 in healthy volunteers. Nephrol Dial Transplant. 2000;15(8):1155–1161. - PubMed
-
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. - PubMed
-
- Torres VE, Meijer E, Bae KT, et al. Rationale and design of the TEMPO (Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes) 3‐4 Study. Am J Kidney Dis. 2011;57(5):692–699. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

