Egress of sperm autoantigen from seminiferous tubules maintains systemic tolerance
- PMID: 28218625
- PMCID: PMC5330742
- DOI: 10.1172/JCI89927
Egress of sperm autoantigen from seminiferous tubules maintains systemic tolerance
Abstract
Autoimmune responses to meiotic germ cell antigens (MGCA) that are expressed on sperm and testis occur in human infertility and after vasectomy. Many MGCA are also expressed as cancer/testis antigens (CTA) in human cancers, but the tolerance status of MGCA has not been investigated. MGCA are considered to be uniformly immunogenic and nontolerogenic, and the prevailing view posits that MGCA are sequestered behind the Sertoli cell barrier in seminiferous tubules. Here, we have shown that only some murine MGCA are sequestered. Nonsequestered MCGA (NS-MGCA) egressed from normal tubules, as evidenced by their ability to interact with systemically injected antibodies and form localized immune complexes outside the Sertoli cell barrier. NS-MGCA derived from cell fragments that were discarded by spermatids during spermiation. They egressed as cargo in residual bodies and maintained Treg-dependent physiological tolerance. In contrast, sequestered MGCA (S-MGCA) were undetectable in residual bodies and were nontolerogenic. Unlike postvasectomy autoantibodies, which have been shown to mainly target S-MGCA, autoantibodies produced by normal mice with transient Treg depletion that developed autoimmune orchitis exclusively targeted NS-MGCA. We conclude that spermiation, a physiological checkpoint in spermatogenesis, determines the egress and tolerogenicity of MGCA. Our findings will affect target antigen selection in testis and sperm autoimmunity and the immune responses to CTA in male cancer patients.
Conflict of interest statement
Figures
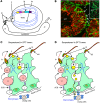

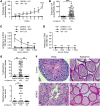
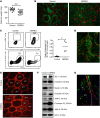

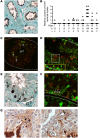
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

