GATA4-dependent organ-specific endothelial differentiation controls liver development and embryonic hematopoiesis
- PMID: 28218627
- PMCID: PMC5330741
- DOI: 10.1172/JCI90086
GATA4-dependent organ-specific endothelial differentiation controls liver development and embryonic hematopoiesis
Abstract
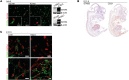
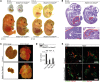
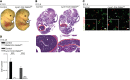
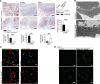
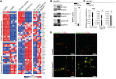
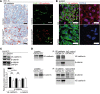
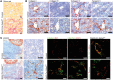
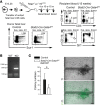
Microvascular endothelial cells (ECs) are increasingly recognized as organ-specific gatekeepers of their microenvironment. Microvascular ECs instruct neighboring cells in their organ-specific vascular niches through angiocrine factors, which include secreted growth factors (angiokines), extracellular matrix molecules, and transmembrane proteins. However, the molecular regulators that drive organ-specific microvascular transcriptional programs and thereby regulate angiodiversity are largely elusive. In contrast to other ECs, which form a continuous cell layer, liver sinusoidal ECs (LSECs) constitute discontinuous, permeable microvessels. Here, we have shown that the transcription factor GATA4 controls murine LSEC specification and function. LSEC-restricted deletion of Gata4 caused transformation of discontinuous liver sinusoids into continuous capillaries. Capillarization was characterized by ectopic basement membrane deposition, formation of a continuous EC layer, and increased expression of VE-cadherin. Correspondingly, ectopic expression of GATA4 in cultured continuous ECs mediated the downregulation of continuous EC-associated transcripts and upregulation of LSEC-associated genes. The switch from discontinuous LSECs to continuous ECs during embryogenesis caused liver hypoplasia, fibrosis, and impaired colonization by hematopoietic progenitor cells, resulting in anemia and embryonic lethality. Thus, GATA4 acts as master regulator of hepatic microvascular specification and acquisition of organ-specific vascular competence, which are indispensable for liver development. The data also establish an essential role of the hepatic microvasculature in embryonic hematopoiesis.
Conflict of interest statement
Figures





Comment in
- Building discontinuous liver sinusoidal vessels doi: 10.1172/JCI92823
References
-
- Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100(2):174–190. doi: 10.1161/01.RES.0000255690.03436.ae. - DOI - PubMed
-
- Lawson ND, et al. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128(19):3675–3683. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

