Mechanisms of Chromosome Congression during Mitosis
- PMID: 28218637
- PMCID: PMC5372006
- DOI: 10.3390/biology6010013
Mechanisms of Chromosome Congression during Mitosis
Abstract
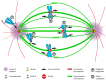
Chromosome congression during prometaphase culminates with the establishment of a metaphase plate, a hallmark of mitosis in metazoans. Classical views resulting from more than 100 years of research on this topic have attempted to explain chromosome congression based on the balance between opposing pulling and/or pushing forces that reach an equilibrium near the spindle equator. However, in mammalian cells, chromosome bi-orientation and force balance at kinetochores are not required for chromosome congression, whereas the mechanisms of chromosome congression are not necessarily involved in the maintenance of chromosome alignment after congression. Thus, chromosome congression and maintenance of alignment are determined by different principles. Moreover, it is now clear that not all chromosomes use the same mechanism for congressing to the spindle equator. Those chromosomes that are favorably positioned between both poles when the nuclear envelope breaks down use the so-called "direct congression" pathway in which chromosomes align after bi-orientation and the establishment of end-on kinetochore-microtubule attachments. This favors the balanced action of kinetochore pulling forces and polar ejection forces along chromosome arms that drive chromosome oscillatory movements during and after congression. The other pathway, which we call "peripheral congression", is independent of end-on kinetochore microtubule-attachments and relies on the dominant and coordinated action of the kinetochore motors Dynein and Centromere Protein E (CENP-E) that mediate the lateral transport of peripheral chromosomes along microtubules, first towards the poles and subsequently towards the equator. How the opposite polarities of kinetochore motors are regulated in space and time to drive congression of peripheral chromosomes only now starts to be understood. This appears to be regulated by position-dependent phosphorylation of both Dynein and CENP-E and by spindle microtubule diversity by means of tubulin post-translational modifications. This so-called "tubulin code" might work as a navigation system that selectively guides kinetochore motors with opposite polarities along specific spindle microtubule populations, ultimately leading to the congression of peripheral chromosomes. We propose an integrated model of chromosome congression in mammalian cells that depends essentially on the following parameters: (1) chromosome position relative to the spindle poles after nuclear envelope breakdown; (2) establishment of stable end-on kinetochore-microtubule attachments and bi-orientation; (3) coordination between kinetochore- and arm-associated motors; and (4) spatial signatures associated with post-translational modifications of specific spindle microtubule populations. The physiological consequences of abnormal chromosome congression, as well as the therapeutic potential of inhibiting chromosome congression are also discussed.
Keywords: CENP-E; Chromokinesin; Dynein; Kinesin; chromosome; kinetochore; microtubule; mitosis; mitotic spindle; polar ejection forces; tubulin code.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Darlington C.D. Recent Advances in Cytology. 2nd ed. The Blakiston Company; Philadelphia, PA, USA: 1937.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

