Capacitive Biosensors and Molecularly Imprinted Electrodes
- PMID: 28218689
- PMCID: PMC5336051
- DOI: 10.3390/s17020390
Capacitive Biosensors and Molecularly Imprinted Electrodes
Abstract
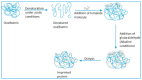
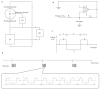
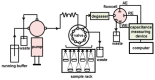
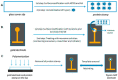
Capacitive biosensors belong to the group of affinity biosensors that operate by registering direct binding between the sensor surface and the target molecule. This type of biosensors measures the changes in dielectric properties and/or thickness of the dielectric layer at the electrolyte/electrode interface. Capacitive biosensors have so far been successfully used for detection of proteins, nucleotides, heavy metals, saccharides, small organic molecules and microbial cells. In recent years, the microcontact imprinting method has been used to create very sensitive and selective biorecognition cavities on surfaces of capacitive electrodes. This chapter summarizes the principle and different applications of capacitive biosensors with an emphasis on microcontact imprinting method with its recent capacitive biosensor applications.
Keywords: affinity biosensors; capacitive biosensors; microcontact imprinting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

