Non-invasive ventilation for cystic fibrosis
- PMID: 28218802
- PMCID: PMC6464053
- DOI: 10.1002/14651858.CD002769.pub5
Non-invasive ventilation for cystic fibrosis
Update in
-
Non-invasive ventilation for cystic fibrosis.Cochrane Database Syst Rev. 2025 Nov 18;11(11):CD002769. doi: 10.1002/14651858.CD002769.pub6. Cochrane Database Syst Rev. 2025. PMID: 41251249
Abstract
Background: Non-invasive ventilation may be a means to temporarily reverse or slow the progression of respiratory failure in cystic fibrosis by providing ventilatory support and avoiding tracheal intubation. Using non-invasive ventilation, in the appropriate situation or individuals, can improve lung mechanics through increasing airflow and gas exchange and decreasing the work of breathing. Non-invasive ventilation thus acts as an external respiratory muscle. This is an update of a previously published review.
Objectives: To compare the effect of non-invasive ventilation versus no non-invasive ventilation in people with cystic fibrosis for airway clearance, during sleep and during exercise.
Search methods: We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Trials Register comprising references identified from comprehensive electronic database searches, handsearching relevant journals and abstract books of conference proceedings. We searched the reference lists of each trial for additional publications possibly containing other trials.Most recent search: 08 August 2016.
Selection criteria: Randomised controlled trials comparing a form of pressure preset or volume preset non-invasive ventilation to no non-invasive ventilation used for airway clearance or during sleep or exercise in people with acute or chronic respiratory failure in cystic fibrosis.
Data collection and analysis: Three reviewers independently assessed trials for inclusion criteria and methodological quality, and extracted data.
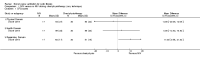
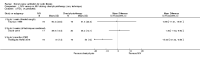
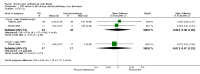
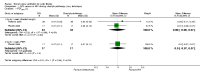
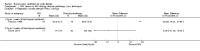
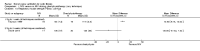
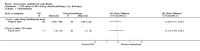
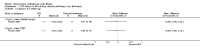
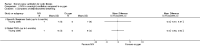
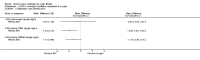
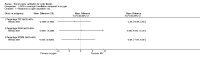
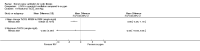
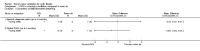
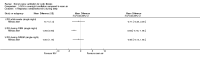
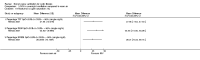
Main results: Ten trials met the inclusion criteria with a total of 191 participants. Seven trials evaluated single treatment sessions, one evaluated a two-week intervention, one evaluated a six-week intervention and one a three-month intervention. It is only possible to blind trials of airway clearance and overnight ventilatory support to the outcome assessors. In most of the trials we judged there was an unclear risk of bias with regards to blinding due to inadequate descriptions. The six-week trial was the only one judged to have a low risk of bias for all other domains. One single intervention trial had a low risk of bias for the randomisation procedure with the remaining trials judged to have an unclear risk of bias. Most trials had a low risk of bias with regard to incomplete outcome data and selective reporting.Six trials (151 participants) evaluated non-invasive ventilation for airway clearance compared with an alternative chest physiotherapy method such as the active cycle of breathing techniques or positive expiratory pressure. Three trials used nasal masks, one used a nasal mask or mouthpiece and one trial used a face mask and in one trial it is unclear. Three of the trials reported on one of the review's primary outcome measures (quality of life). Results for the reviews secondary outcomes showed that airway clearance may be easier with non-invasive ventilation and people with cystic fibrosis may prefer it. We were unable to find any evidence that non-invasive ventilation increases sputum expectoration, but it did improve some lung function parameters.Three trials (27 participants) evaluated non-invasive ventilation for overnight ventilatory support compared to oxygen or room air using nasal masks (two trials) and nasal masks or full face masks (one trial). Trials reported on two of the review's primary outcomes (quality of life and symptoms of sleep-disordered breathing). Results for the reviews secondary outcome measures showed that they measured lung function, gas exchange, adherence to treatment and preference, and nocturnal transcutaneous carbon dioxide. Due to the small numbers of participants and statistical issues, there were discrepancies in the results between the RevMan and the original trial analyses. No clear differences were found between non-invasive ventilation compared with oxygen or room air except for exercise performance, which significantly improved with non-invasive ventilation compared to room air over six weeks.One trial (13 participants) evaluated non-invasive ventilation on exercise capacity (interface used was unclear) and did not reported on any of the review's primary outcomes. The trial found no clear differences between non-invasive ventilation compared to no non-invasive ventilation for any of our outcomes.Three trials reported on adverse effects. One trial, evaluating non-invasive ventilation for airway clearance, reported that a participant withdrew at the start of the trial due to pain on respiratory muscle testing. One trial evaluating non-invasive ventilation for overnight support reported that one participant could not tolerate an increase in inspiratory positive airway pressure. A second trial evaluating non-invasive ventilation in this setting reported that one participant did not tolerate the non-invasive ventilation mask, one participant developed a pneumothorax when breathing room air and two participants experienced aerophagia which resolved when inspiratory positive airway pressure was decreased.
Authors' conclusions: Non-invasive ventilation may be a useful adjunct to other airway clearance techniques, particularly in people with cystic fibrosis who have difficulty expectorating sputum. Non-invasive ventilation, used in addition to oxygen, may improve gas exchange during sleep to a greater extent than oxygen therapy alone in moderate to severe disease. The effect of NIV on exercise is unclear. These benefits of non-invasive ventilation have largely been demonstrated in single treatment sessions with small numbers of participants. The impact of this therapy on pulmonary exacerbations and disease progression remain unclear. There is a need for long-term randomised controlled trials which are adequately powered to determine the clinical effects of non-invasive ventilation in cystic fibrosis airway clearance and exercise.
Conflict of interest statement
Amanda Piper was a clinical consultant to ResMed Australia until 2004. She has also been involved in: educational activities sponsored by manufacturers of bilevel devices (Mayo Healthcare, Australia; Weinmann, Germany; Air Liquide, Australia; ResMed, Australia; Philps Respironics, Australia) and transcutaneous carbon dioxide devices (Sentec, Switzwerland; industry‐sponsored research (ResMed, Australia) and has received equipment for research projects from distributors of bilevel equipment (Air Liquide, Australia; Mayo Healthcare, Australia) as well as a competitive research grant from the ResMed Foundation.
Fidelma Moran and Judy Bradley received a NIPPY3 ventilator for a research clinical trial from Respicare.
Figures
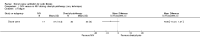


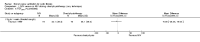

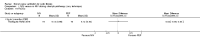

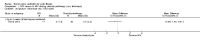


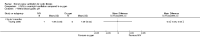
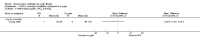







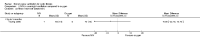
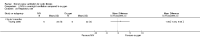
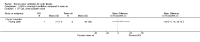
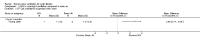


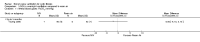

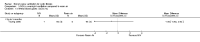
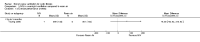







Update of
-
Non-invasive ventilation for cystic fibrosis.Cochrane Database Syst Rev. 2013 Apr 30;(4):CD002769. doi: 10.1002/14651858.CD002769.pub4. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2017 Feb 20;2:CD002769. doi: 10.1002/14651858.CD002769.pub5. PMID: 23633308 Updated.
References
References to studies included in this review
Dwyer 2015 {published data only}
-
- Dwyer TJ, Robbins L, Kelly P, Piper AJ, Bell SC, Bye PT. Non‐invasive ventilation used as an adjunct to airway clearance treatments improves lung function during an acute exacerbation of cystic fibrosis: a randomised trial. Journal of Physiotherapy 2015;61(3):142‐7. [CENTRAL: 1104106; CFGD Register: OV22; CRS: 5500135000001431; PUBMED: 26096013] - PubMed
Fauroux 1999 {published data only}
-
- Fauroux B, Boule M, Escurat G, Pouzet C, Harf A, Isabey D. Chest physiotherapy in cystic fibrosis: improvement of tolerance with nasal pressure support ventilation [abstract]. European Respiratory Journal. Supplement. 1997;10 Suppl 25:79S. [CENTRAL: 382789; CFGD Register: PE103b; CRS: 5500050000000431]
-
- Fauroux B, Boule M, Lofaso F, Zerah F, Clement A, Harf A, et al. Chest physiotherapy in cystic fibrosis: improved tolerance with nasal pressure support ventilation. Pediatrics 1999;103(3):E32. [CFGD Register: PE103a] - PubMed
Gozal 1997 {published data only}
-
- Gozal D. Nocturnal ventilatory support in patients with cystic fibrosis: comparison with supplemental oxygen. European Respiratory Journal 1997;10(9):1999‐2003. [CFGD jRegister: OV10] - PubMed
Holland 2003 {published data only}
-
- Holland A, Denehey L, Ntoumenopoulos G, Wilson J. Non‐invasive ventilation prevents inspiratory muscle fatigue and oxygen desaturation during airway clearance in adults with exacerbations of cystic fibrosis [abstract]. Proceedings of the American Thoracic Society 99th International Conference; 2003 May 16‐21; Seattle; USA. 2003:D041. [CFGD Register: PE143b]
-
- Holland A, Denehy L, Ntoumenopoulos G, McMeeken J, Wilson J. Non‐invasive ventilation prevents inspiratory muscle fatigue and oxygen desaturation during airway clearance in adults with acute exacerbations of cystic fibrosis [abstract]. Proceedings of the Thoracic Society of Australia & New Zealand Annual Scientific Meeting; 2003 April 4‐9; Adelaide, Australia. 2003:Abst #P140. [CFGD Register: PE143d]
-
- Holland A, Denehy L, Ntoumenopoulos G, Naughton M, Wilson J. Non‐invasive ventilation prevents inspiratory muscle fatigue and oxygen desaturation during airway clearance in adults with acute exacerbations of cystic fibrosis [abstract]. Journal of Cystic Fibrosis 2003;2(Suppl 1):S62. [CFGD Register: PE143a]
-
- Holland A, Denehy L, Ntoumenopoulos G, Naughton M, et al. Non‐invasive ventilation preserves inspiratory muscle strength and prevents oxygen desaturation during airway clearance in adults with exacerbations of cystic fibrosis [abstract]. Australian Journal of Physiotherapy 2004;50(3):A6. [CENTRAL: 547610; CFGD Register: PE143e; CRS: 5500050000000411]
Kofler 1998 {published data only}
-
- Kofler AM, Carlesi A, Cutrera R, Leone P, Lucidi V, Rosati S, et al. BiPAP versus PEP as chest physiotherapy in patients with cystic fibrosis [abstract]. Pediatric Pulmonology 1998;26(Suppl 17):344. [CFGD Register: PE95]
Lima 2014 {published data only}
-
- Lima C, Andrade AD, Rattes C, Campos S, Brandao D, Aliverti A, et al. Effect of noninvasive ventilation on functional exercise capacity, lung function and compartmental chest wall volume in children with cystic fibrosis. European Respiratory Journal 2013;42 Suppl 57:P5065. [CENTRAL: 1099891; CFGD Register: PE216b ; CRS: 5500050000000267; EMBASE: 71843506]
-
- Lima CA, Andrade ADFD, Campos SL, Brandao DC, Fregonezi G, Mourato IP, et al. Effects of noninvasive ventilation on treadmill 6‐min walk distance and regional chest wall volumes in cystic fibrosis: randomized controlled trial. Respiratory Medicine 2014;108(10):1460‐8. [CENTRAL: 1022461; CFGD Register: PE216a; CRS: 5500050000000162; EMBASE: 2014860248] - PubMed
Milross 2001 {published and unpublished data}
-
- Milross MA, Piper AJ, Norman M, Becker HF, Willson GN, Grunstein RR, et al. Low‐flow oxygen and bilevel ventilatory support. American Journal of Respiratory and Critical Care Medicine 2001;163(1):129‐34. [CFGD Register: OV8b] - PubMed
-
- Milross MA, Piper AJ, Norman M, Willson GN, Becker HF, Grunstein RR, et al. The effects of low flow oxygen and bilevel ventilatory support on gas exchange and ventilation during sleep in patients with cystic fibrosis [abstract]. Pediatric Pulmonology 1999;28(Suppl 19):285. [CFGD Register: OV8a]
Placidi 2006 {published data only}
-
- Placidi G, Cornacchia M, Cappelletti LM, Mastella G, Assale BM, Braggion C. Short‐term effects of positive airway pressure on sputum clearance by directed coughing: a cross‐over randomized study [abstract]. Pediatric Pulmonology 2001;32(Suppl 22):313. [CFGD Register: PE128a]
-
- Placidi G, Cornacchia M, Polese G, Zanolla L, Assael B, Braggion C. Chest physiotherapy with positive airway pressure: a pilot study of short‐term effects on sputum clearance in patients with cystic fibrosis and severe airway obstruction. Respiratory Care 2006;51(10):1145‐53. [CFGD Register: PE128b] - PubMed
Rodriguez Hortal 2016 {published data only}
-
- Rodriguez Hortal MC, Hjelte L. Non invasive ventilation as airway clearance technique compared to PEP in adult patients with cystic fibrosis [abstract]. Journal of Cystic Fibrosis 2013;12 Suppl 1:S18, Abstract no: WS9.4. [CENTRAL: 875000; CFGD Register: PE203a; CRS: 5500100000011654]
Young 2008 {published data only}
-
- Young AC, Wilson JW, Kotsimbos TC, Naughton MT. Online Data Supplement to 'Randomised placebo controlled trial of non‐invasive ventilation for hypercapnia in cystic fibrosis' [online]. Thorax 2008;63(1):72‐7 online. [CFGD Register: OV19c] - PubMed
-
- Young AC, Wilson JW, Kotsimbos TC, Naughton MT. Randomised placebo controlled trial of non‐invasive ventilation for hypercapnia in cystic fibrosis. Thorax 2008;63(1):72‐7. [CFGD Register: OV19b] - PubMed
-
- Young AC, Wilson JW, Kotsimbos TC, Naughton MT. Randomized placebo‐controlled trial of non‐invasive ventilation for hypercapnia in cystic fibrosis [abstract]. Proceedings of the Japanese Society of Sleep Research Conference. 2006:A9‐10. [CFGD Register: OV19a] - PubMed
References to studies excluded from this review
Elkins 2004 {published data only}
-
- Elkins MR, Eberl S, Alsion J, Bye P. The effect of bi‐level non‐invasive ventilation on mucociliary clearance in subjects with cystic fibrosis [abstract].. Pediatric Pulmonology. 2004; Vol. 38(Suppl 27):315. [CFGD Register: OV20]
Falk 2006 {published data only}
-
- Falk B, Nini A, Zigel L, Yahav Y, Aviram M, Rivlin J, et al. Effect of low altitude at the Dead Sea on exercise capacity and cardiopulmonary response to exercise in cystic fibrosis patients with moderate to severe lung disease. Pediatric Pulmonology 2006;41(3):234‐41. [CFGD Register: OV13] - PubMed
Fauroux 2000a {published data only}
-
- Fauroux B, Itti E, Pigeot J, Isabey D, Meignan M, Ferry G, et al. Optimization of aerosol deposition by pressure support in children with cystic fibrosis: an experimental and clinical study. American Journal of Respiratory and Critical Care Medicine 2000;162(6):2265‐71. [CFGD Register: DT21] - PubMed
Fauroux 2000b {published data only}
-
- Faurox B, Pigeot J, Isabey D, Harf A, Clèment A, Lofaso F. Two modes of non invasive mechanical ventilation can decrease the work of breathing in cystic fibrosis children with an acute exacerbation. European Respiratory Journal 2000;16(Suppl 31):15s. [CENTRAL: 415631; CFGD Register: OV23; CRS: 5500050000000270]
Fauroux 2001 {published data only}
-
- Fauroux B, Pigeot J, Isabey D, Clement A, Lofaso F. In vivo physiologic comparison of two ventilators used for domiciliary ventilation in children with cystic fibrosis. Critical Care Medicine 2001;29(11):2097‐105. [CFGD Register: PH137] - PubMed
Fauroux 2004 {published data only}
-
- Fauroux B, Louis B, Essouri S, Leroux K, Lofaso F. Back up rate during non invasive mechanical ventilation in cystic fibrosis [abstract]. European Respiratory Journal 2003;22(Suppl 45):262s. [CFGD Register: PH157b]
-
- Fauroux B, Louis B, Hart N, Essouri S, Leroux K, Clement A, et al. The effect of back‐up rate during non‐invasive ventilation in young patients with cystic fibrosis. Intensive Care Medicine 2004;30(4):673‐81. [CFGD Register: PH157a] - PubMed
Greenough 2004 {published data only}
-
- Greenough A, Limb E, Marlow N, Peacock JL, Calvert S. Radiological outcome of very prematurely born infants randomised to high frequency oscillatory or conventional ventilation. European Journal of Pediatrics 2004;163(11):671‐4. - PubMed
Parreira 2008 {published data only}
-
- Parreira V, Pires S, Sulmonett N, Camargos P, Haddad J, Britto R. Positive expiratory pressure and lung function in cystic fibrosis patients [abstract]. Proceedings of the European Respiratory Society Annual Congress; 2008 Oct 4‐8; Berlin, Germany.. 2008:E1779. [CENTRAL: 679902; CFGD Register: PE212; CRS: 5500050000000051]
Piper 1992 {published data only}
-
- Piper AJ, Parker S, Torzillo PJ, Sullivan CE, Bye PTP. Nocturnal nasal IPPV stabilizes patients with cystic fibrosis and hypercapnic respiratory failure. Chest 1992;102(3):846‐50. - PubMed
Regnis 1994 {published data only}
-
- Regnis JA, Piper AJ, Henke KG, Parker S, Bye PTP, Sullivan CE. Benefits of nocturnal nasal CPAP in patients with cystic fibrosis. Chest 1994;106(6):1717‐24. - PubMed
Riethmueller 2006 {published data only}
-
- Riethmueller J, Borth‐Bruhns T, Kumpf M, Vonthein R, Wiskirchen J, Stern M, et al. Recombinant human deoxyribonuclease shortens ventilation time in young, mechanically ventilated children.. Pediatric Pulmonology 2006;41(1):61‐6. [CFGD Register: BD125] - PubMed
Serra 2000 {published data only}
-
- Serra A, Appendini L, Braggion C, Mastella G, Rossi A, Polese G. Noninvasive proportional assist ventilation (PAV) and pressure support ventilation (PSV) in patients with cystic fibrosis (CF): an overnight study [abstract]. American Journal of Respiratory and Critical Care Medicine 2000;161(Suppl 3):A555. [CFGD Register: OV16]
References to studies awaiting assessment
Petrone 2009 {published data only}
-
- Petrone A, Quartieri M, Tirone F, Tirone C, Mauro GF. Management of patients with cystic fibrosis: Role of non invasive ventilation (NIV) and chest physiotherapy [abstract]. Proceedings of European Respiratory Society Annual Congress; 2010 Sep 18‐22; Barcelona, Spain. 2010:[E3907]. [CENTRAL: 777469; CFGD Register: PE220b; CRS: 5500050000000268]
-
- Petrone A, Quartieri M, Tolisano A, Tirone C, Tirone F. Non invasive ventilation (NIV) assists chest physiotherapy for treatment of patients with cystic fibrosis [abstract]. Proceedings of the European Respiratory Society Annual Congress; 2009 Sep 12‐16; Vienna, Austria. 2009:[P1262]. [CENTRAL: 758852; CFGD Register: PE220a ; CRS: 5500050000000269]
Additional references
Ballard 1996
-
- Ballard RD, Sutarik JM, Clover CW, Suh BY. Effects of non‐REM sleep on ventilation and respiratory mechanics in adults with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 1996;153(1):266‐71. - PubMed
Bott 2009
-
- Bott J, Blumenthal S, Buxton M, Ellum S, Falconer C, Garrod R, et al. Guidelines for the physiotherapy management of the adult, medical spontaneously breathing patient. Thorax 2009;64(Suppl 1):i1‐i51. - PubMed
Curtin 2002
-
- Curtin F, Altman DG, Elbourne D. Meta‐analysis combining parallel and cross over trials. Statistics in Medicine 2002;21(15):2131‐44. - PubMed
Davidson 2016
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Epidemiological Association 2002;31(1):140‐9. - PubMed
Fuchs 1994
-
- Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW, et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. New England Journal of Medicine 1994;331(10):637‐42. - PubMed
Higgins 2003
Hodson 1991
-
- Hodson ME, Madden BP, Steven MH, Tsang VT, Yacoub MH. Non‐invasive mechanical ventilation for CF patients ‐ a potential bridge to transplantation. European Respiratory Journal 1991;4(5):524‐7. - PubMed
Jüni 2001
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Stata 2001 [Computer program]
-
- Stata Corporation. Stata. Statistical Software. Version 7.0. College Station, Texas: Stata Corporation, 2001.
UK CF Trust 2011
-
- UK CF Trust. Standards for the clinical care of children and adults with CF in the UK 2011. www.cysticfibrosis.org.uk/the‐work‐we‐do/clinical‐care/consensus‐documents (accessed 07 July 2016).
Yankaskas 1999
-
- Yankaskas JR, Egan TM, Mauro MA. Major Complications. In: Yankaskas JR, Knowles MR editor(s). Cystic Fibrosis in Adults. Philadelphia: Lippincott‐Raven, 1999:175‐94.
Zinman 1989
-
- Zinman R, Corey M, Coates AL, Canny GJ, Connolly J, Levison H, et al. Nocturnal home oxygen in the treatment of hypoxaemic cystic fibrosis patients. Journal of Pediatrics 1989;114(3):368‐77. - PubMed
References to other published versions of this review
Moran 2003
Moran 2007
Moran 2011
-
- Moran F, Bradley J, Piper AJ. Non‐invasive ventilation for cystic fibrosis. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD002769.pub3] - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

