Cell-matrix signals specify bone endothelial cells during developmental osteogenesis
- PMID: 28218908
- PMCID: PMC5580829
- DOI: 10.1038/ncb3476
Cell-matrix signals specify bone endothelial cells during developmental osteogenesis
Abstract
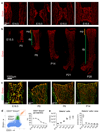
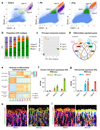
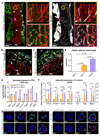
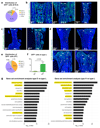
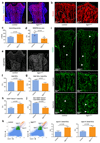
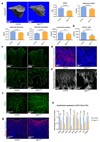
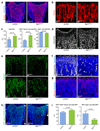
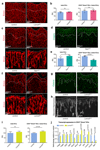
Blood vessels in the mammalian skeletal system control bone formation and support haematopoiesis by generating local niche environments. While a specialized capillary subtype, termed type H, has been recently shown to couple angiogenesis and osteogenesis in adolescent, adult and ageing mice, little is known about the formation of specific endothelial cell populations during early developmental endochondral bone formation. Here, we report that embryonic and early postnatal long bone contains a specialized endothelial cell subtype, termed type E, which strongly supports osteoblast lineage cells and later gives rise to other endothelial cell subpopulations. The differentiation and functional properties of bone endothelial cells require cell-matrix signalling interactions. Loss of endothelial integrin β1 leads to endothelial cell differentiation defects and impaired postnatal bone growth, which is, in part, phenocopied by endothelial cell-specific laminin α5 mutants. Our work outlines fundamental principles of vessel formation and endothelial cell differentiation in the developing skeletal system.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures
References
-
- Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. - PubMed
-
- Eshkar-Oren I, et al. The forming limb skeleton serves as a signaling center for limb vasculature patterning via regulation of Vegf. Development. 2009;136:1263–1272. - PubMed
-
- Maes C, et al. Impaired angiogenesis and endochondral bone formation in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Mech Dev. 2002;111:61–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

