Rationale for stimulator of interferon genes-targeted cancer immunotherapy
- PMID: 28219022
- PMCID: PMC5362072
- DOI: 10.1016/j.ejca.2016.12.028
Rationale for stimulator of interferon genes-targeted cancer immunotherapy
Abstract
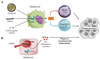
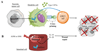
The efficacy of checkpoint inhibitor therapy illustrates that cancer immunotherapy, which aims to foster the host immune response against cancer to achieve durable anticancer responses, can be successfully implemented in a routine clinical practice. However, a substantial proportion of patients does not benefit from this treatment, underscoring the need to identify alternative strategies to defeat cancer. Despite the demonstration in the 1990's that the detection of danger signals, including the nucleic acids DNA and RNA, by dendritic cells (DCs) in a cancer setting is essential for eliciting host defence, the molecular sensors responsible for recognising these danger signals and eliciting anticancer immune responses remain incompletely characterised, possibly explaining the disappointing results obtained so far upon the clinical implementation of DC-based cancer vaccines. In 2008, STING (stimulator of interferon genes), was identified as a protein that is indispensable for the recognition of cytosolic DNA. The central role of STING in controlling anticancer immune responses was exemplified by observations that spontaneous and radiation-induced adaptive anticancer immunity was reduced in the absence of STING, illustrating the potential of STING-targeting for cancer immunotherapy. Here, we will discuss the relevance of manipulating the STING signalling pathway for cancer treatment and integrating STING-targeting based strategies into combinatorial therapies to obtain long-lasting anticancer immune responses.
Keywords: Adaptive immunity; Anticancer therapies; Cancer immunotherapy; DNA; Danger signal; Innate immunity; STING.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures


References
-
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. - PubMed
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–52. - PubMed
-
- Desmet CJ, Ishii KJ. Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nat Rev Immunol. 2012;12(7):479–91. - PubMed
-
- Barbalat R, Ewald SE, Mouchess ML, Barton GM. Nucleic acid recognition by the innate immune system. Annu Rev Immunol. 2011;29:185–214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

