Substance P activates Mas-related G protein-coupled receptors to induce itch
- PMID: 28219706
- PMCID: PMC5546940
- DOI: 10.1016/j.jaci.2016.12.980
Substance P activates Mas-related G protein-coupled receptors to induce itch
Abstract
Background: Substance P (SP) is linked to itch and inflammation through activation of receptors on mast cells and sensory neurons. There is increasing evidence that SP functions through Mas-related G protein-coupled receptors (Mrgprs) in addition to its conventional receptor, neurokinin-1.
Objective: Because Mrgprs mediate some aspects of inflammation that had been considered mediated by neurokinin-1 receptor (NK-1R), we sought to determine whether itch induced by SP can also be mediated by Mrgprs.
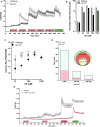
Methods: Genetic and pharmacologic approaches were used to evaluate the contribution of Mrgprs to SP-induced scratching behavior and activation of cultured dorsal root ganglion neurons from mice.
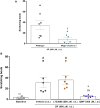
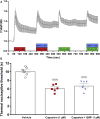
Results: SP-induced scratching behavior and activation of cultured dorsal root ganglion neurons was dependent on Mrgprs rather than NK-1R.
Conclusion: We deduce that SP activates MrgprA1 on sensory neurons rather than NK-1R to induce itch.
Keywords: Mas-related G protein–coupled receptors; Substance P; calcium imaging; dorsal root ganglion neurons; knockout mice; receptor antagonist.
Copyright © 2017 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Figures

References
-
- Duval A, Dubertret L. Aprepitant as an antipruritic agent? N Engl J Med. 2009;361:1415–6. - PubMed
-
- Santini D, Vincenzi B, Guida FM, Imperatori M, Schiavon G, Venditti O, et al. Aprepitant for management of severe pruritus related to biological cancer treatments: a pilot study. Lancet Oncol. 2012;13:1020–4. - PubMed
-
- Wallengren J. Topical aprepitant in clinical and experimental pruritus. Arch Dermatol. 2012;148:957–9. - PubMed
-
- Wallengren J, Edvinsson L. Topical non-peptide antagonists of sensory neurotransmitters substance P and CGRP do not modify patch test and prick test reactions: a vehicle-controlled, double-blind pilot study. Arch Dermatol Res. 2014;306:505–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

