Peroxiredoxin 1 (Prx1) is a dual-function enzyme by possessing Cys-independent catalase-like activity
- PMID: 28219939
- PMCID: PMC5452528
- DOI: 10.1042/BCJ20160851
Peroxiredoxin 1 (Prx1) is a dual-function enzyme by possessing Cys-independent catalase-like activity
Abstract
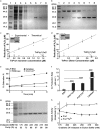
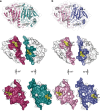
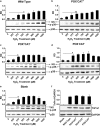
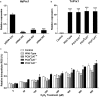
Peroxiredoxin (Prx) was previously known as a Cys-dependent thioredoxin. However, we unexpectedly observed that Prx1 from the green spotted puffer fish Tetraodon nigroviridis (TnPrx1) was able to reduce H2O2 in a manner independent of Cys peroxidation and reductants. This study aimed to validate a novel function for Prx1, delineate the biochemical features and explore its antioxidant role in cells. We have confirmed that Prx1 from the puffer fish and humans truly possesses a catalase (CAT)-like activity that is independent of Cys residues and reductants, but dependent on iron. We have identified that the GVL motif was essential to the CAT-like activity of Prx1, but not to the Cys-dependent thioredoxin peroxidase (POX) activity, and generated mutants lacking POX and/or CAT-like activities for individual functional validation. We discovered that the TnPrx1 POX and CAT-like activities possessed different kinetic features in the reduction of H2O2 The overexpression of wild-type TnPrx1 and mutants differentially regulated the intracellular levels of reactive oxygen species (ROS) and the phosphorylation of p38 in HEK-293T cells treated with H2O2 Prx1 is a dual-function enzyme by acting as POX and CAT with varied affinities towards ROS. This study extends our knowledge on Prx1 and provides new opportunities to further study the biological roles of this family of antioxidants.
Keywords: ROS; catalase-like activity; peroxiredoxin 1 (Prx1); puffer fish.
© 2017 The Author(s).
Conflict of interest statement
The Authors declare that there are no competing interests associated with the manuscript.
Figures






References
-
- Rhee S. G., Kang S. W., Jeong W., Chang T.-S., Yang K.-S. and Woo H. A. (2005) Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr. Opin. Cell Biol. 17, 183–189 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

