Enhanced Sensitivity to Hyperpolarizing Inhibition in Mesoaccumbal Relative to Nigrostriatal Dopamine Neuron Subpopulations
- PMID: 28219982
- PMCID: PMC5373120
- DOI: 10.1523/JNEUROSCI.2969-16.2017
Enhanced Sensitivity to Hyperpolarizing Inhibition in Mesoaccumbal Relative to Nigrostriatal Dopamine Neuron Subpopulations
Abstract
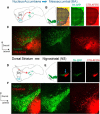
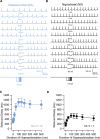
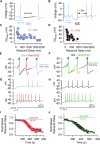
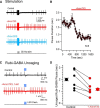
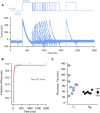
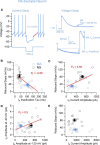
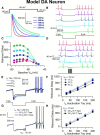
Midbrain dopamine neurons recorded in vivo pause their firing in response to reward omission and aversive stimuli. While the initiation of pauses typically involves synaptic or modulatory input, intrinsic membrane properties may also enhance or limit hyperpolarization, raising the question of how intrinsic conductances shape pauses in dopamine neurons. Using retrograde labeling and electrophysiological techniques combined with computational modeling, we examined the intrinsic conductances that shape pauses evoked by current injections and synaptic stimulation in subpopulations of dopamine neurons grouped according to their axonal projections to the nucleus accumbens or dorsal striatum in mice. Testing across a range of conditions and pulse durations, we found that mesoaccumbal and nigrostriatal neurons differ substantially in rebound properties with mesoaccumbal neurons displaying significantly longer delays to spiking following hyperpolarization. The underlying mechanism involves an inactivating potassium (IA) current with decay time constants of up to 225 ms, and small-amplitude hyperpolarization-activated currents (IH), characteristics that were most often observed in mesoaccumbal neurons. Pharmacological block of IA completely abolished rebound delays and, importantly, shortened synaptically evoked inhibitory pauses, thereby demonstrating the involvement of A-type potassium channels in prolonging pauses evoked by GABAergic inhibition. Therefore, these results show that mesoaccumbal and nigrostriatal neurons display differential responses to hyperpolarizing inhibitory stimuli that favors a higher sensitivity to inhibition in mesoaccumbal neurons. These findings may explain, in part, observations from in vivo experiments that ventral tegmental area neurons tend to exhibit longer aversive pauses relative to SNc neurons.SIGNIFICANCE STATEMENT Our study examines rebound, postburst, and synaptically evoked inhibitory pauses in subpopulations of midbrain dopamine neurons. We show that pauses in dopamine neuron firing, evoked by either stimulation of GABAergic inputs or hyperpolarizing current injections, are enhanced by a subclass of potassium conductances that are recruited at voltages below spike threshold. Importantly, A-type potassium currents recorded in mesoaccumbal neurons displayed substantially slower inactivation kinetics, which, combined with weaker expression of hyperpolarization-activated currents, lengthened hyperpolarization-induced delays in spiking relative to nigrostriatal neurons. These results suggest that input integration differs among dopamine neurons favoring higher sensitivity to inhibition in mesoaccumbal neurons and may partially explain in vivo observations that ventral tegmental area neurons exhibit longer aversive pauses relative to SNc neurons.
Keywords: action potential; dopamine; mesoaccumbal; nigrostriatal; potassium channel.
Copyright © 2017 the authors 0270-6474/17/373311-20$15.00/0.
Figures




Similar articles
-
Dopamine Inhibition Differentially Controls Excitability of Substantia Nigra Dopamine Neuron Subpopulations through T-Type Calcium Channels.J Neurosci. 2017 Mar 29;37(13):3704-3720. doi: 10.1523/JNEUROSCI.0117-17.2017. Epub 2017 Mar 6. J Neurosci. 2017. PMID: 28264982 Free PMC article.
-
Dopamine modulation of two subthreshold currents produces phase shifts in activity of an identified motoneuron.J Neurophysiol. 1995 Oct;74(4):1404-20. doi: 10.1152/jn.1995.74.4.1404. J Neurophysiol. 1995. PMID: 8989381
-
In vivo functional diversity of midbrain dopamine neurons within identified axonal projections.Elife. 2019 Oct 3;8:e48408. doi: 10.7554/eLife.48408. Elife. 2019. PMID: 31580257 Free PMC article.
-
GABAergic control of substantia nigra dopaminergic neurons.Prog Brain Res. 2007;160:189-208. doi: 10.1016/S0079-6123(06)60011-3. Prog Brain Res. 2007. PMID: 17499115 Review.
-
Dissecting the diversity of midbrain dopamine neurons.Trends Neurosci. 2013 Jun;36(6):336-42. doi: 10.1016/j.tins.2013.03.003. Epub 2013 Apr 12. Trends Neurosci. 2013. PMID: 23582338 Review.
Cited by
-
Kv4 channel expression and kinetics in GABAergic and non-GABAergic rNST neurons.J Neurophysiol. 2020 Dec 1;124(6):1727-1742. doi: 10.1152/jn.00396.2020. Epub 2020 Sep 30. J Neurophysiol. 2020. PMID: 32997557 Free PMC article.
-
Estrous Cycle Mediates Midbrain Neuron Excitability Altering Social Behavior upon Stress.J Neurosci. 2023 Feb 1;43(5):736-748. doi: 10.1523/JNEUROSCI.1504-22.2022. Epub 2022 Dec 22. J Neurosci. 2023. PMID: 36549906 Free PMC article.
-
VTA dopamine neurons are hyperexcitable in 3xTg-AD mice due to casein kinase 2-dependent SK channel dysfunction.Nat Commun. 2024 Nov 8;15(1):9673. doi: 10.1038/s41467-024-53891-1. Nat Commun. 2024. PMID: 39516200 Free PMC article.
-
VTA dopamine neurons are hyperexcitable in 3xTg-AD mice due to casein kinase 2-dependent SK channel dysfunction.bioRxiv [Preprint]. 2023 Nov 17:2023.11.16.567486. doi: 10.1101/2023.11.16.567486. bioRxiv. 2023. Update in: Nat Commun. 2024 Nov 8;15(1):9673. doi: 10.1038/s41467-024-53891-1. PMID: 38014232 Free PMC article. Updated. Preprint.
-
Muscarinic Receptor Activation Preferentially Inhibits Rebound in Vulnerable Dopaminergic Neurons.J Neurosci. 2025 Apr 16;45(16):e1443242025. doi: 10.1523/JNEUROSCI.1443-24.2025. J Neurosci. 2025. PMID: 40000233
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
