ACTH Receptor (MC2R) Specificity: What Do We Know About Underlying Molecular Mechanisms?
- PMID: 28220105
- PMCID: PMC5292628
- DOI: 10.3389/fendo.2017.00013
ACTH Receptor (MC2R) Specificity: What Do We Know About Underlying Molecular Mechanisms?
Abstract
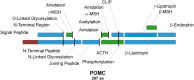
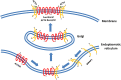
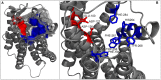
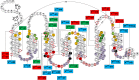
Coincidentally, the release of this Research Topic in Frontiers in Endocrinology takes place 25 years after the discovery of the adrenocorticotropic hormone receptor (ACTHR) by Mountjoy and colleagues. In subsequent years, following the discovery of other types of mammalian melanocortin receptors (MCRs), ACTHR also became known as melanocortin type 2 receptor (MC2R). At present, five types of MCRs have been reported, all of which share significant sequence similarity at the amino acid level, and all of which specifically bind melanocortins (MCs)-a group of biologically active peptides generated by proteolysis of the proopiomelanocortin precursor. All MCs share an identical -H-F-R-W- pharmacophore sequence. α-Melanocyte-stimulating hormone (α-MSH) and adrenocorticotropic hormone (ACTH) are the most extensively studied MCs and are derived from the same region. Essentially, α-MSH is formed from the first 13 amino acid residues of ACTH. ACTHR is unique among MCRs because it binds one sole ligand-ACTH, which makes it a very attractive research object for molecular pharmacologists. However, much research has failed, and functional studies of this receptor are lagging behind other MCRs. The reason for these difficulties has already been outlined by Mountjoy and colleagues in their publication on ACTHR coding sequence discovery where the Cloudman S91 melanoma cell line was used for receptor expression because it was a "more sensitive assay system." Subsequent work showed that ACTHR could be successfully expressed only in endogenous MCR-expressing cell lines, since in other cell lines it is retained within the endoplasmic reticulum. The resolution of this methodological problem came in 2005 with the discovery of melanocortin receptor accessory protein, which is required for the formation of functionally active ACTHR. The decade that followed this discovery was filled with exciting research that provided insight into the molecular mechanisms underlying the action of ACTHR. The purpose of this review is to summarize the advances in this fascinating research field.
Keywords: ACTHR; MC2R; mutagenesis; mutation; site directed; specificity.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

