CpG-ODN Shapes Alum Adjuvant Activity Signaling via MyD88 and IL-10
- PMID: 28220116
- PMCID: PMC5289984
- DOI: 10.3389/fimmu.2017.00047
CpG-ODN Shapes Alum Adjuvant Activity Signaling via MyD88 and IL-10
Abstract
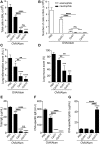
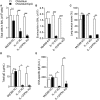
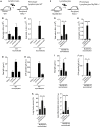
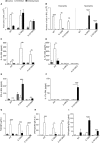
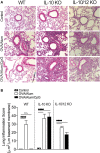
Aluminum-containing adjuvants usually referred as Alum are considered as T helper type-2 (Th2) adjuvants, while agonists of toll-like receptors (TLRs) are viewed as adjuvants that favor Th1/Th17 immunity. Alum has been used in numerous vaccine formulations; however, its undesired pro-Th2 adjuvant activity constitutes a caveat for Alum-based vaccines. Combining Alum with TLR-dependent, pro-Th1/Th17 adjuvants might dampen the pro-Th2 activity and improve the effectiveness of vaccine formulations. Here, using the ovalbumin (OVA) model of allergic lung inflammation, we found that sensitization with the synthetic TLR9 agonist, which is composed of oligodeoxynucleotides containing CpG motifs adsorbed to Alum, inhibited the development of OVA-induced lung allergic Th2 responses without shifting toward a Th1 pattern. The conversion of T cell immunity from the polarized allergic Th2 response to a non-polarized form by sensitization with OVA/Alum/CpG was dependent on MyD88 signaling in myeloid cells. Notably, sensitization of IL-10-deficient mice with OVA/Alum/CpG resulted in the development of neutrophilic lung inflammation associated with IFNγ production. However, in IL-10/IL-12-deficient mice, it resulted in neutrophilic inflammation dominated by IL-17 production. We conclude that OVA/Alum/CpG sensitization signaling via MyD88 and IL-10 molecules results in non-polarized immunity. Conversely, OVA/Alum/CpG sensitization in presence of MyD88 but absence of IL-10 or IL-10/IL-12 molecules results, respectively, in neutrophilic inflammation associated with IFNγ or IL-17 production. Our work provides novel OVA models of lung inflammation and suggests that Alum/CpG-based formulations might be of potential use in anti-allergic or anti-infectious processes.
Keywords: Alum; CpG-ODN; OVA model; T helper cells; TLR agonists; adjuvants; lung inflammation.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources

