Increased mercury emissions from modern dental amalgams
- PMID: 28220332
- PMCID: PMC5352807
- DOI: 10.1007/s10534-017-0004-3
Increased mercury emissions from modern dental amalgams
Abstract
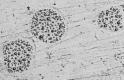
All types of dental amalgams contain mercury, which partly is emitted as mercury vapor. All types of dental amalgams corrode after being placed in the oral cavity. Modern high copper amalgams exhibit two new traits of increased instability. Firstly, when subjected to wear/polishing, droplets rich in mercury are formed on the surface, showing that mercury is not being strongly bonded to the base or alloy metals. Secondly, high copper amalgams emit substantially larger amounts of mercury vapor than the low copper amalgams used before the 1970s. High copper amalgams has been developed with focus on mechanical strength and corrosion resistance, but has been sub-optimized in other aspects, resulting in increased instability and higher emission of mercury vapor. This has not been presented to policy makers and scientists. Both low and high copper amalgams undergo a transformation process for several years after placement, resulting in a substantial reduction in mercury content, but there exist no limit for maximum allowed emission of mercury from dental amalgams. These modern high copper amalgams are nowadays totally dominating the European, US and other markets, resulting in significant emissions of mercury, not considered when judging their suitability for dental restoration.
Keywords: Copper amalgam; Mercury; Non-gamma-two; Non-ɣ2.
Conflict of interest statement
The authors have no conflicting interests.
Figures

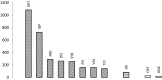
References
-
- Anusavice KJ, Shen C, Rawls HR (2012) Phillips’ Science of Dental Materials, 12th edn. Elsevier, St. Louis
-
- Fredin B. Mercury release from dental amalgam fillings. Int J Risk Saf Med. 1994;4(3):197–208. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

