Chronic alcohol feeding potentiates hormone-induced calcium signalling in hepatocytes
- PMID: 28220501
- PMCID: PMC5430227
- DOI: 10.1113/JP273891
Chronic alcohol feeding potentiates hormone-induced calcium signalling in hepatocytes
Abstract
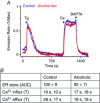
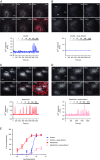
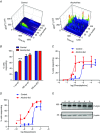
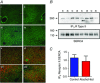
Chronic alcohol consumption causes a spectrum of liver diseases, but the pathogenic mechanisms driving the onset and progression of disease are not clearly defined. We show that chronic alcohol feeding sensitizes rat hepatocytes to Ca2+ -mobilizing hormones resulting in a leftward shift in the concentration-response relationship and the transition from oscillatory to more sustained and prolonged Ca2+ increases. Our data demonstrate that alcohol-dependent adaptation in the Ca2+ signalling pathway occurs at the level of hormone-induced inositol 1,4,5 trisphosphate (IP3 ) production and does not involve changes in the sensitivity of the IP3 receptor or size of internal Ca2+ stores. We suggest that prolonged and aberrant hormone-evoked Ca2+ increases may stimulate the production of mitochondrial reactive oxygen species and contribute to alcohol-induced hepatocyte injury. ABSTRACT: 'Adaptive' responses of the liver to chronic alcohol consumption may underlie the development of cell and tissue injury. Alcohol administration can perturb multiple signalling pathways including phosphoinositide-dependent cytosolic calcium ([Ca2+ ]i ) increases, which can adversely affect mitochondrial Ca2+ levels, reactive oxygen species production and energy metabolism. Our data indicate that chronic alcohol feeding induces a leftward shift in the dose-response for Ca2+ -mobilizing hormones resulting in more sustained and prolonged [Ca2+ ]i increases in both cultured hepatocytes and hepatocytes within the intact perfused liver. Ca2+ increases were initiated at lower hormone concentrations, and intercellular calcium wave propagation rates were faster in alcoholics compared to controls. Acute alcohol treatment (25 mm) completely inhibited hormone-induced calcium increases in control livers, but not after chronic alcohol-feeding, suggesting desensitization to the inhibitory actions of ethanol. Hormone-induced inositol 1,4,5 trisphosphate (IP3 ) accumulation and phospholipase C (PLC) activity were significantly potentiated in hepatocytes from alcohol-fed rats compared to controls. Removal of extracellular calcium, or chelation of intracellular calcium did not normalize the differences in hormone-stimulated PLC activity, indicating calcium-dependent PLCs are not upregulated by alcohol. We propose that the liver 'adapts' to chronic alcohol exposure by increasing hormone-dependent IP3 formation, leading to aberrant calcium increases, which may contribute to hepatocyte injury.
Keywords: alcohol; calcium signalling; hepatocyte.
© 2017 The Authors. The Journal of Physiology © 2017 The Physiological Society.
Figures






Comment in
-
Alcohol and calcium make a potent cocktail.J Physiol. 2017 May 15;595(10):3109-3110. doi: 10.1113/JP274133. Epub 2017 Apr 18. J Physiol. 2017. PMID: 28295353 Free PMC article. No abstract available.
Similar articles
-
Ethanol Disrupts Hormone-Induced Calcium Signaling in Liver.Function (Oxf). 2021 Jan 8;2(2):zqab002. doi: 10.1093/function/zqab002. eCollection 2021. Function (Oxf). 2021. PMID: 33604575 Free PMC article.
-
The effect of chronic alcohol consumption on mitochondrial calcium handling in hepatocytes.Biochem J. 2016 Nov 1;473(21):3903-3921. doi: 10.1042/BCJ20160255. Epub 2016 Aug 31. Biochem J. 2016. PMID: 27582500 Free PMC article.
-
Differential Regulation of Multiple Steps in Inositol 1,4,5-Trisphosphate Signaling by Protein Kinase C Shapes Hormone-stimulated Ca2+ Oscillations.J Biol Chem. 2015 Jul 24;290(30):18519-33. doi: 10.1074/jbc.M115.657767. Epub 2015 Jun 15. J Biol Chem. 2015. PMID: 26078455 Free PMC article.
-
Hierarchical organization of calcium signals in hepatocytes: from experiments to models.Biochim Biophys Acta. 2000 Dec 20;1498(2-3):134-52. doi: 10.1016/s0167-4889(00)00090-2. Biochim Biophys Acta. 2000. PMID: 11108957 Review.
-
A review of the role of reactive oxygen and nitrogen species in alcohol-induced mitochondrial dysfunction.Free Radic Res. 2003 Jun;37(6):585-96. doi: 10.1080/1071576031000091711. Free Radic Res. 2003. PMID: 12868485 Review.
Cited by
-
Pathways of topological rank analysis (PoTRA): a novel method to detect pathways involved in hepatocellular carcinoma.PeerJ. 2018 Apr 9;6:e4571. doi: 10.7717/peerj.4571. eCollection 2018. PeerJ. 2018. PMID: 29666752 Free PMC article.
-
Ethanol Disrupts Hormone-Induced Calcium Signaling in Liver.Function (Oxf). 2021 Jan 8;2(2):zqab002. doi: 10.1093/function/zqab002. eCollection 2021. Function (Oxf). 2021. PMID: 33604575 Free PMC article.
-
Changes in the microstructure of compact and trabecular bone tissues of mice subchronically exposed to alcohol.J Biol Res (Thessalon). 2018 May 24;25:8. doi: 10.1186/s40709-018-0079-1. eCollection 2018 Dec. J Biol Res (Thessalon). 2018. PMID: 29876325 Free PMC article.
-
Causality Analysis and Cell Network Modeling of Spatial Calcium Signaling Patterns in Liver Lobules.Front Physiol. 2018 Oct 4;9:1377. doi: 10.3389/fphys.2018.01377. eCollection 2018. Front Physiol. 2018. PMID: 30337879 Free PMC article.
-
Binge drinking triggers VGLUT3-mediated glutamate secretion and subsequent hepatic inflammation by activating mGluR5/NOX2 in Kupffer cells.Nat Commun. 2025 Jul 1;16(1):5546. doi: 10.1038/s41467-025-60820-3. Nat Commun. 2025. PMID: 40595616 Free PMC article.
References
-
- Abou Hashieh I, Mathieu S, Besson F & Gerolami A (1996). Inhibition of gap junction intercellular communications of cultured rat hepatocytes by ethanol: role of ethanol metabolism. J Hepatol 24, 360–367. - PubMed
-
- Albano E (2008a). New concepts in the pathogenesis of alcoholic liver disease. Expert Rev Gastroenterol Hepatol 2, 749–759. - PubMed
-
- Albano E (2008b). Oxidative mechanisms in the pathogenesis of alcoholic liver disease. Mol Aspects Med 29, 9–16. - PubMed
-
- Altamirano J & Bataller R (2011). Alcoholic liver disease: pathogenesis and new targets for therapy. Nat Rev Gastroenterol Hepatol 8, 491–501. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

