Guanosine tetraphosphate modulates salicylic acid signalling and the resistance of Arabidopsis thaliana to Turnip mosaic virus
- PMID: 28220595
- PMCID: PMC6638062
- DOI: 10.1111/mpp.12548
Guanosine tetraphosphate modulates salicylic acid signalling and the resistance of Arabidopsis thaliana to Turnip mosaic virus
Abstract
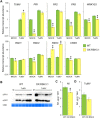
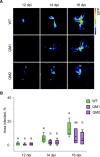
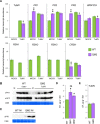
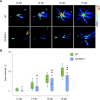
Chloroplasts can act as key players in the perception and acclimatization of plants to incoming environmental signals. A growing body of evidence indicates that chloroplasts play a critical role in plant immunity. Chloroplast function can be regulated by the nucleotides guanosine tetraphosphate and pentaphosphate [(p)ppGpp]. In plants, (p)ppGpp levels increase in response to abiotic stress and to plant hormones which are involved in abiotic and biotic stress signalling. In this study, we analysed the transcriptome of Arabidopsis plants that over-accumulate (p)ppGpp, and unexpectedly found a decrease in the levels of a broad range of transcripts for plant defence and immunity. To determine whether (p)ppGpp is involved in the modulation of plant immunity, we analysed the susceptibility of plants with different levels of (p)ppGpp to Turnip mosaic virus (TuMV) carrying a green fluorescent protein (GFP) reporter. We found that (p)ppGpp accumulation was associated with increased susceptibility to TuMV and reduced levels of the defence hormone salicylic acid (SA). In contrast, plants with lower (p)ppGpp levels showed reduced susceptibility to TuMV, and this was associated with the precocious up-regulation of defence-related genes and increased SA content. We have therefore demonstrated a new link between (p)ppGpp metabolism and plant immunity in Arabidopsis.
Keywords: (p)ppGpp; TuMV; Turnip mosaic virus; chloroplast; guanosine tetraphosphate; pathogen; salicylic acid.
© 2017 BSPP AND JOHN WILEY & SONS LTD.
Figures

References
-
- Aznar, A. , Chen, N.W. , Rigault, M. , Riache, N. , Joseph, D. , Desmaele, D. , Mouille, G. , Boutet, S. , Soubigou‐Taconnat, L. , Renou, J.P. , Thomine, S. , Expert, D. and Dellagi, A. (2014) Scavenging iron: a novel mechanism of plant immunity activation by microbial siderophores. Plant Physiol. 164, 2167–2183. - PMC - PubMed
-
- Bailey, K. , Cevik, V. , Holton, N. , Byrne‐Richardson, J. , Sohn, K.H. , Coates, M. , Woods‐Tör, A. , Aksoy, H.M. , Hughes, L. , Baxter, L. , Jones, J.D. , Beynon, J. , Holub, E.B. and Tör, M. (2011) Molecular cloning of ATR5(Emoy2) from Hyaloperonospora arabidopsidis, an avirulence determinant that triggers RPP5‐mediated defense in Arabidopsis. Mol. Plant–Microbe Interact. 24, 827–838. - PubMed
-
- Beauchemin, C. , Bougie, V. and Laliberte, J.F. (2005) Simultaneous production of two foreign proteins from a polyvirus‐based vector. Virus Res. 112, 1–8. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

