High performance communication by people with paralysis using an intracortical brain-computer interface
- PMID: 28220753
- PMCID: PMC5319839
- DOI: 10.7554/eLife.18554
High performance communication by people with paralysis using an intracortical brain-computer interface
Abstract
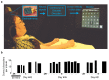
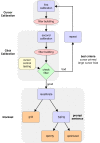
Brain-computer interfaces (BCIs) have the potential to restore communication for people with tetraplegia and anarthria by translating neural activity into control signals for assistive communication devices. While previous pre-clinical and clinical studies have demonstrated promising proofs-of-concept (Serruya et al., 2002; Simeral et al., 2011; Bacher et al., 2015; Nuyujukian et al., 2015; Aflalo et al., 2015; Gilja et al., 2015; Jarosiewicz et al., 2015; Wolpaw et al., 1998; Hwang et al., 2012; Spüler et al., 2012; Leuthardt et al., 2004; Taylor et al., 2002; Schalk et al., 2008; Moran, 2010; Brunner et al., 2011; Wang et al., 2013; Townsend and Platsko, 2016; Vansteensel et al., 2016; Nuyujukian et al., 2016; Carmena et al., 2003; Musallam et al., 2004; Santhanam et al., 2006; Hochberg et al., 2006; Ganguly et al., 2011; O'Doherty et al., 2011; Gilja et al., 2012), the performance of human clinical BCI systems is not yet high enough to support widespread adoption by people with physical limitations of speech. Here we report a high-performance intracortical BCI (iBCI) for communication, which was tested by three clinical trial participants with paralysis. The system leveraged advances in decoder design developed in prior pre-clinical and clinical studies (Gilja et al., 2015; Kao et al., 2016; Gilja et al., 2012). For all three participants, performance exceeded previous iBCIs (Bacher et al., 2015; Jarosiewicz et al., 2015) as measured by typing rate (by a factor of 1.4-4.2) and information throughput (by a factor of 2.2-4.0). This high level of performance demonstrates the potential utility of iBCIs as powerful assistive communication devices for people with limited motor function.Clinical Trial No: NCT00912041.
Keywords: ALS; assistive technology; brain-machine interface; human; human biology; medicine; neural prosthesis; neuroscience.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures

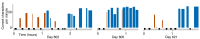







References
-
- Bacher D, Jarosiewicz B, Masse NY, Stavisky SD, Simeral JD, Newell K, Oakley EM, Cash SS, Friehs G, Hochberg LR. Neural Point-and-Click communication by a person with incomplete Locked-In Syndrome. Neurorehabilitation and Neural Repair. 2015;29:462–471. doi: 10.1177/1545968314554624. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

