Aptamers for respiratory syncytial virus detection
- PMID: 28220811
- PMCID: PMC5318870
- DOI: 10.1038/srep42794
Aptamers for respiratory syncytial virus detection
Abstract
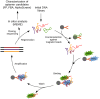
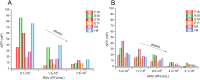
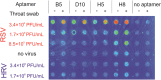
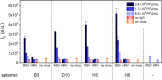
The identification of the infectious agents is pivotal for appropriate care of patients with viral diseases. Current viral diagnostics rely on selective detection of viral nucleic acid or protein components. In general, detection of proteins rather than nucleic acids is technically more suitable for rapid tests. However, protein-based virus identification methods depend on antibodies limiting the practical applicability of these approaches. Aptamers rival antibodies in target selectivity and binding affinity, and excel in terms of robustness and cost of synthesis. Although aptamers have been generated for virus identification in laboratory settings, their introduction into routine virus diagnostics has not been realized, yet. Here, we demonstrate that the rationally designed SELEX protocol can be applied on whole virus to select aptamers, which can potentially be applied for viral diagnostics. This approach does not require purified virus protein or complicated virus purification. The presented data also illustrate that corroborating the functionality of aptamers with various approaches is essential to pinpoint the most appropriate aptamer amongst the panel of candidates obtained by the selection. Our protocol yielded aptamers capable of detecting respiratory syncytial virus (RSV), an important pathogen causing severe disease especially in young infants, at clinically relevant concentrations in complex matrices.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Hall C. B. In Principles and Practice of Clinical Virology 323–341 (John Wiley & Sons, Ltd.), doi: 10.1002/0470020970.ch7 (2004). - DOI
-
- Henderson F. W., Collier A. M., Clyde W. A. & Denny F. W. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N. Engl. J. Med. 300, 530–4 (1979). - PubMed
-
- Storch G. A. Diagnostic virology. Clin. Infect. Dis. 31, 739–51 (2000). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

