A novel role of the organizer gene Goosecoid as an inhibitor of Wnt/PCP-mediated convergent extension in Xenopus and mouse
- PMID: 28220837
- PMCID: PMC5318956
- DOI: 10.1038/srep43010
A novel role of the organizer gene Goosecoid as an inhibitor of Wnt/PCP-mediated convergent extension in Xenopus and mouse
Abstract
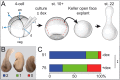
Goosecoid (Gsc) expression marks the primary embryonic organizer in vertebrates and beyond. While functions have been assigned during later embryogenesis, the role of Gsc in the organizer has remained enigmatic. Using conditional gain-of-function approaches in Xenopus and mouse to maintain Gsc expression in the organizer and along the axial midline, neural tube closure defects (NTDs) arose and dorsal extension was compromised. Both phenotypes represent convergent extension (CE) defects, arising from impaired Wnt/planar cell polarity (PCP) signaling. Dvl2 recruitment to the cell membrane was inhibited by Gsc in Xenopus animal cap assays and key Wnt/PCP factors (RhoA, Vangl2, Prickle, Wnt11) rescued Gsc-mediated NTDs. Re-evaluation of endogenous Gsc functions in MO-mediated gene knockdown frog and knockout mouse embryos unearthed PCP/CE-related phenotypes as well, including cartilage defects in Xenopus and misalignment of inner ear hair cells in mouse. Our results assign a novel function to Gsc as an inhibitor of Wnt/PCP-mediated CE. We propose that in the organizer Gsc represses CE as well: Gsc-expressing prechordal cells, which leave the organizer first, migrate and do not undergo CE like the Gsc-negative notochordal cells, which subsequently emerge from the organizer. In this model, Gsc provides a switch between cell migration and CE, i.e. cell intercalation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures






Similar articles
-
Siamois and Twin are redundant and essential in formation of the Spemann organizer.Dev Biol. 2011 Apr 15;352(2):367-81. doi: 10.1016/j.ydbio.2011.01.034. Epub 2011 Feb 3. Dev Biol. 2011. PMID: 21295564 Free PMC article.
-
Transcriptional integration of Wnt and Nodal pathways in establishment of the Spemann organizer.Dev Biol. 2012 Aug 15;368(2):231-41. doi: 10.1016/j.ydbio.2012.05.018. Epub 2012 May 22. Dev Biol. 2012. PMID: 22627292 Free PMC article.
-
NEDD4L regulates convergent extension movements in Xenopus embryos via Disheveled-mediated non-canonical Wnt signaling.Dev Biol. 2014 Aug 1;392(1):15-25. doi: 10.1016/j.ydbio.2014.05.003. Epub 2014 May 14. Dev Biol. 2014. PMID: 24833518
-
TRPM6 and TRPM7: Novel players in cell intercalation during vertebrate embryonic development.Dev Dyn. 2020 Aug;249(8):912-923. doi: 10.1002/dvdy.182. Epub 2020 May 26. Dev Dyn. 2020. PMID: 32315468 Review.
-
Xenopus neural tube closure: A vertebrate model linking planar cell polarity to actomyosin contractions.Curr Top Dev Biol. 2021;145:41-60. doi: 10.1016/bs.ctdb.2021.04.001. Epub 2021 May 4. Curr Top Dev Biol. 2021. PMID: 34074535 Review.
Cited by
-
Functions of the FGF signalling pathway in cephalochordates provide insight into the evolution of the prechordal plate.Development. 2022 May 15;149(10):dev200252. doi: 10.1242/dev.200252. Epub 2022 May 16. Development. 2022. PMID: 35575387 Free PMC article.
-
Furry is required for cell movements during gastrulation and functionally interacts with NDR1.Sci Rep. 2021 Mar 23;11(1):6607. doi: 10.1038/s41598-021-86153-x. Sci Rep. 2021. PMID: 33758327 Free PMC article.
-
Goosecoid Controls Neuroectoderm Specification via Dual Circuits of Direct Repression and Indirect Stimulation in Xenopus Embryos.Mol Cells. 2021 Oct 31;44(10):723-735. doi: 10.14348/molcells.2021.0055. Mol Cells. 2021. PMID: 34711690 Free PMC article.
-
Insights into the Etiology of Mammalian Neural Tube Closure Defects from Developmental, Genetic and Evolutionary Studies.J Dev Biol. 2018 Aug 21;6(3):22. doi: 10.3390/jdb6030022. J Dev Biol. 2018. PMID: 30134561 Free PMC article. Review.
-
NSD1 Mutations in Sotos Syndrome Induce Differential Expression of Long Noncoding RNAs, miR646 and Genes Controlling the G2/M Checkpoint.Life (Basel). 2022 Jul 2;12(7):988. doi: 10.3390/life12070988. Life (Basel). 2022. PMID: 35888078 Free PMC article.
References
-
- Ninomiya H., Elinson R. P. & Winklbauer R. Antero-posterior tissue polarity links mesoderm convergent extension to axial patterning. Nature 430, 364–367 (2004). - PubMed
-
- Blum M. et al. Ciliation and gene expression distinguish between node and posterior notochord in the mammalian embryo. Differentiation 75, 133–146 (2007). - PubMed
-
- Spemann H. & Mangold H. Über Induktion von Embryonalanlagen durch Implantation artfremder Organisatoren. Archiv für Entwicklungsmechanik der Organismen 100, 599–638 (1924).
-
- Blum M. et al. Gastrulation in the mouse: the role of the homeobox gene goosecoid. Cell 69, 1097–1106 (1992). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

