Group A Streptococcal M1 Protein Provides Resistance against the Antimicrobial Activity of Histones
- PMID: 28220899
- PMCID: PMC5318940
- DOI: 10.1038/srep43039
Group A Streptococcal M1 Protein Provides Resistance against the Antimicrobial Activity of Histones
Abstract
Histones are essential elements of chromatin structure and gene regulation in eukaryotes. An unexpected attribute of these nuclear proteins is their antimicrobial activity. A framework for histone release and function in host defense in vivo was revealed with the discovery of neutrophil extracellular traps, a specialized cell death process in which DNA-based structures containing histones are extruded to ensnare and kill bacteria. Investigating the susceptibility of various Gram-positive pathogens to histones, we found high-level resistance by one leading human pathogen, group A Streptococcus (GAS). A screen of isogenic mutants revealed that the highly surface-expressed M1 protein, a classical GAS virulence factor, was required for high-level histone resistance. Biochemical and microscopic analyses revealed that the N-terminal domain of M1 protein binds and inactivates histones before they reach their cell wall target of action. This finding illustrates a new pathogenic function for this classic GAS virulence factor, and highlights a potential innate immune evasion strategy that may be employed by other bacterial pathogens.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

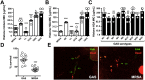
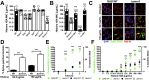
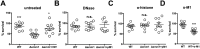

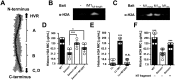
Similar articles
-
Group A Streptococcal M1 Protein Sequesters Cathelicidin to Evade Innate Immune Killing.Cell Host Microbe. 2015 Oct 14;18(4):471-7. doi: 10.1016/j.chom.2015.09.004. Cell Host Microbe. 2015. PMID: 26468750 Free PMC article.
-
Virulence Role of the GlcNAc Side Chain of the Lancefield Cell Wall Carbohydrate Antigen in Non-M1-Serotype Group A Streptococcus.mBio. 2018 Jan 30;9(1):e02294-17. doi: 10.1128/mBio.02294-17. mBio. 2018. PMID: 29382733 Free PMC article.
-
M1 protein allows Group A streptococcal survival in phagocyte extracellular traps through cathelicidin inhibition.J Innate Immun. 2009;1(3):202-14. doi: 10.1159/000203645. Epub 2009 Feb 20. J Innate Immun. 2009. PMID: 20375578 Free PMC article.
-
The nonideal coiled coil of M protein and its multifarious functions in pathogenesis.Adv Exp Med Biol. 2011;715:197-211. doi: 10.1007/978-94-007-0940-9_12. Adv Exp Med Biol. 2011. PMID: 21557065 Free PMC article. Review.
-
[Mechanisms of immune evasion by Streptococcus pyogenes].Tanpakushitsu Kakusan Koso. 2009 Jun;54(8 Suppl):982-7. Tanpakushitsu Kakusan Koso. 2009. PMID: 21089528 Review. Japanese. No abstract available.
Cited by
-
Antibiotic Treatment, Mechanisms for Failure, and Adjunctive Therapies for Infections by Group A Streptococcus.Front Microbiol. 2021 Nov 4;12:760255. doi: 10.3389/fmicb.2021.760255. eCollection 2021. Front Microbiol. 2021. PMID: 34803985 Free PMC article. Review.
-
Combination of lidocaine gel and povidone-iodine to decrease acquired infections in procedures performed using topical anesthesia.J Cataract Refract Surg. 2020 Jul;46(7):1047-1050. doi: 10.1097/j.jcrs.0000000000000245. J Cataract Refract Surg. 2020. PMID: 32427643 Free PMC article.
-
Fibronectin-binding protein B (FnBPB) from Staphylococcus aureus protects against the antimicrobial activity of histones.J Biol Chem. 2019 Mar 8;294(10):3588-3602. doi: 10.1074/jbc.RA118.005707. Epub 2019 Jan 8. J Biol Chem. 2019. PMID: 30622139 Free PMC article.
-
Lipid droplets and the host-pathogen dynamic: FATal attraction?J Cell Biol. 2021 Aug 2;220(8):e202104005. doi: 10.1083/jcb.202104005. Epub 2021 Jun 24. J Cell Biol. 2021. PMID: 34165498 Free PMC article. Review.
-
Playing With Fire: Proinflammatory Virulence Mechanisms of Group A Streptococcus.Front Cell Infect Microbiol. 2021 Jul 6;11:704099. doi: 10.3389/fcimb.2021.704099. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34295841 Free PMC article. Review.
References
-
- Kossel A. Ueber einen peptonartigen Bestandteil des Zellkerns. Z Physiol Chem 8, 511–515 (1884).
-
- Stedman E. Cell specificity of histones. Nature 166, 780–781 (1950). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

