Membrane cholesterol access into a G-protein-coupled receptor
- PMID: 28220900
- PMCID: PMC5321766
- DOI: 10.1038/ncomms14505
Membrane cholesterol access into a G-protein-coupled receptor
Abstract
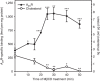
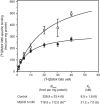
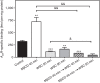
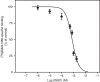
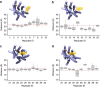
Cholesterol is a key component of cell membranes with a proven modulatory role on the function and ligand-binding properties of G-protein-coupled receptors (GPCRs). Crystal structures of prototypical GPCRs such as the adenosine A2A receptor (A2AR) have confirmed that cholesterol finds stable binding sites at the receptor surface suggesting an allosteric role of this lipid. Here we combine experimental and computational approaches to show that cholesterol can spontaneously enter the A2AR-binding pocket from the membrane milieu using the same portal gate previously suggested for opsin ligands. We confirm the presence of cholesterol inside the receptor by chemical modification of the A2AR interior in a biotinylation assay. Overall, we show that cholesterol's impact on A2AR-binding affinity goes beyond pure allosteric modulation and unveils a new interaction mode between cholesterol and the A2AR that could potentially apply to other GPCRs.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
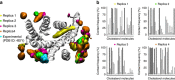

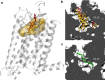

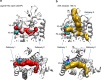
References
-
- Paila Y. D. & Chattopadhyay A. The function of G-protein coupled receptors and membrane cholesterol: specific or general interaction? Glycoconj. J. 26, 711–720 (2009). - PubMed
-
- Paila Y. D. & Chattopadhyay A. Membrane cholesterol in the function and organization of G-protein coupled receptors. Subcell. Biochem 51, 381–398 (2010). - PubMed
-
- Oates J. & Watts A. Uncovering the intimate relationship between lipids, cholesterol and GPCR activation. Curr. Opin. Struct. Biol. 21, 802–807 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

