Hormonal Maintenance Therapy for Women With Low-Grade Serous Cancer of the Ovary or Peritoneum
- PMID: 28221866
- PMCID: PMC5455356
- DOI: 10.1200/JCO.2016.71.0632
Hormonal Maintenance Therapy for Women With Low-Grade Serous Cancer of the Ovary or Peritoneum
Abstract
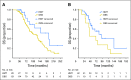
Purpose The purpose of this study was to examine outcomes associated with hormonal maintenance therapy (HMT) compared with routine observation (OBS) after primary cytoreductive surgery and platinum-based chemotherapy in women with stage II to IV low-grade serous carcinoma of the ovary or peritoneum. Patients and Methods Eligibility criteria for patients from our database were: treatment with primary surgery followed by platinum-based chemotherapy, stage II to IV disease, at least 2 years of follow-up for patients who had not experienced recurrence, and adequate clinical information. The two groups were compared for progression-free survival (PFS) and overall survival, and a multivariable Cox regression analysis was performed. Subset analyses for patients who were disease free or had persistent disease were also performed. Results Between 1981 and 2013, 203 eligible patients-133 who underwent OBS and 70 who received HMT-were seen at our institution. Median PFS for patients who underwent OBS was 26.4 months, compared with 64.9 months for those who received HMT ( P < .001). No statistically significant difference in overall survival was observed between the two groups (102.7 v 115.7 months, respectively). For subgroups of women who were disease free or had persistent disease, median PFS was superior for those who received HMT (81.1 v 30.0 months; P < .001 and 38.1 v 15.2 months; P < .001, respectively). Women who received HMT had a significantly lower risk of disease progression compared with those who underwent OBS (hazard ratio, 0.44; 95% CI, 0.31 to 0.64; P < .001). Conclusion Women with stage II to IV low-grade serous carcinoma who received HMT after primary treatment had significantly longer PFS compared with women who underwent OBS. These findings warrant further investigation using a prospective trial design.
Figures


References
-
- Gershenson DM, Sun CC, Lu KH, et al. Clinical behavior of stage II-IV low-grade serous carcinoma of the ovary. Obstet Gynecol. 2006;108:361–368. - PubMed
-
- Schmeler KM, Sun CC, Bodurka DC, et al. Neoadjuvant chemotherapy for low-grade serous carcinoma of the ovary or peritoneum. Gynecol Oncol. 2008;108:510–514. - PubMed
-
- Gershenson DM, Sun CC, Bodurka D, et al. Recurrent low-grade serous ovarian carcinoma is relatively chemoresistant. Gynecol Oncol. 2009;114:48–52. - PubMed
-
- Schmeler KM, Sun CC, Malpica A, et al. Low-grade serous primary peritoneal carcinoma. Gynecol Oncol. 2011;121:482–486. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

