Two dynamic regimes in the human gut microbiome
- PMID: 28222117
- PMCID: PMC5340412
- DOI: 10.1371/journal.pcbi.1005364
Two dynamic regimes in the human gut microbiome
Abstract
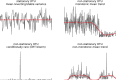
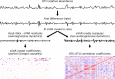
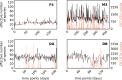
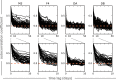
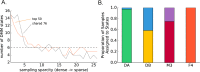
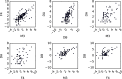
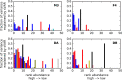
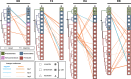
The gut microbiome is a dynamic system that changes with host development, health, behavior, diet, and microbe-microbe interactions. Prior work on gut microbial time series has largely focused on autoregressive models (e.g. Lotka-Volterra). However, we show that most of the variance in microbial time series is non-autoregressive. In addition, we show how community state-clustering is flawed when it comes to characterizing within-host dynamics and that more continuous methods are required. Most organisms exhibited stable, mean-reverting behavior suggestive of fixed carrying capacities and abundant taxa were largely shared across individuals. This mean-reverting behavior allowed us to apply sparse vector autoregression (sVAR)-a multivariate method developed for econometrics-to model the autoregressive component of gut community dynamics. We find a strong phylogenetic signal in the non-autoregressive co-variance from our sVAR model residuals, which suggests niche filtering. We show how changes in diet are also non-autoregressive and that Operational Taxonomic Units strongly correlated with dietary variables have much less of an autoregressive component to their variance, which suggests that diet is a major driver of microbial dynamics. Autoregressive variance appears to be driven by multi-day recovery from frequent facultative anaerobe blooms, which may be driven by fluctuations in luminal redox. Overall, we identify two dynamic regimes within the human gut microbiota: one likely driven by external environmental fluctuations, and the other by internal processes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

