RhoA promotes epidermal stem cell proliferation via PKN1-cyclin D1 signaling
- PMID: 28222172
- PMCID: PMC5319766
- DOI: 10.1371/journal.pone.0172613
RhoA promotes epidermal stem cell proliferation via PKN1-cyclin D1 signaling
Abstract
Objective: Epidermal stem cells (ESCs) play a critical role in wound healing, but the mechanism underlying ESC proliferation is not well defined. Here, we explore the effects of RhoA on ESC proliferation and the possible underlying mechanism.
Methods: Human ESCs were enriched by rapid adhesion to collagen IV. RhoA(+/+)(G14V), RhoA(-/-)(T19N) and pGFP control plasmids were transfected into human ESCs. The effect of RhoA on cell proliferation was detected by cell proliferation and DNA synthesis assays. Induction of PKN1 activity by RhoA was determined by immunoblot analysis, and the effects of PKN1 on RhoA in terms of inducing cell proliferation and cyclin D1 expression were detected using specific siRNA targeting PKN1. The effects of U-46619 (a RhoA agonist) and C3 transferase (a RhoA antagonist) on ESC proliferation were observed in vivo.
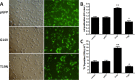
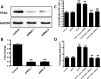
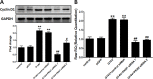
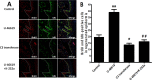
Results: RhoA had a positive effect on ESC proliferation, and PKN1 activity was up-regulated by the active RhoA mutant (G14V) and suppressed by RhoA T19N. Moreover, the ability of RhoA to promote ESC proliferation and DNA synthesis was interrupted by PKN1 siRNA. Additionally, cyclin D1 protein and mRNA expression levels were up-regulated by RhoA G14V, and these effects were inhibited by siRNA-mediated knock-down of PKN1. RhoA also promoted ESC proliferation via PKN in vivo.
Conclusion: This study shows that the effect of RhoA on ESC proliferation is mediated by activation of the PKN1-cyclin D1 pathway in vitro, suggesting that RhoA may serve as a new therapeutic target for wound healing.
Conflict of interest statement
Figures


Similar articles
-
RhoA signaling modulates cyclin D1 expression in human lung fibroblasts; implications for idiopathic pulmonary fibrosis.Respir Res. 2006 Jun 15;7(1):88. doi: 10.1186/1465-9921-7-88. Respir Res. 2006. PMID: 16776827 Free PMC article.
-
Nitric oxide promotes epidermal stem cell proliferation via FOXG1-c-Myc signalling.Nitric Oxide. 2018 Feb 28;73:1-8. doi: 10.1016/j.niox.2017.12.002. Epub 2017 Dec 14. Nitric Oxide. 2018. PMID: 29248687
-
Role of small GTPase Rho in regulating corneal epithelial wound healing.Invest Ophthalmol Vis Sci. 2008 Mar;49(3):900-9. doi: 10.1167/iovs.07-1122. Invest Ophthalmol Vis Sci. 2008. PMID: 18326710 Free PMC article.
-
Signal transduction mediated by cyclin D1: from mitogens to cell proliferation: a molecular target with therapeutic potential.Cancer Treat Res. 2004;119:217-37. doi: 10.1007/1-4020-7847-1_11. Cancer Treat Res. 2004. PMID: 15164880 Review. No abstract available.
-
COVID-19 and primary wound healing: A new insights and advance.Int Wound J. 2023 Dec;20(10):4422-4428. doi: 10.1111/iwj.14324. Epub 2023 Jul 24. Int Wound J. 2023. PMID: 37488776 Free PMC article. Review.
Cited by
-
GDF-5 induces epidermal stem cell migration via RhoA-MMP9 signalling.J Cell Mol Med. 2021 Feb;25(4):1939-1948. doi: 10.1111/jcmm.15925. Epub 2020 Dec 27. J Cell Mol Med. 2021. PMID: 33369147 Free PMC article.
-
Annexin A1 protects epidermal stem cells against ultraviolet-B irradiation-induced mitochondrial dysfunction.Arch Dermatol Res. 2024 Jun 14;316(7):385. doi: 10.1007/s00403-024-02875-8. Arch Dermatol Res. 2024. PMID: 38874830
-
The Rho GTPase exchange factor Vav2 promotes extensive age-dependent rewiring of the hair follicle stem cell transcriptome.Front Cell Dev Biol. 2023 Sep 26;11:1252834. doi: 10.3389/fcell.2023.1252834. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37822868 Free PMC article.
-
Quantifying the Dynamics of Hematopoiesis by In Vivo IdU Pulse-Chase, Mass Cytometry, and Mathematical Modeling.Cytometry A. 2019 Oct;95(10):1075-1084. doi: 10.1002/cyto.a.23799. Epub 2019 May 31. Cytometry A. 2019. PMID: 31150166 Free PMC article.
-
GDF-5 promotes epidermal stem cells proliferation via Foxg1-cyclin D1 signaling.Stem Cell Res Ther. 2021 Jan 7;12(1):42. doi: 10.1186/s13287-020-02106-7. Stem Cell Res Ther. 2021. PMID: 33413682 Free PMC article.
References
-
- Chai L, Cao C, Bi S, Dai X, Gan L, Guo R, et al. (2010) Small Rho GTPase Rac1 determines human epidermal stem cell fate in vitro. Int J Mol Med 25: 723–727. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

