Pre-existing neutralizing antibody mitigates B cell dysregulation and enhances the Env-specific antibody response in SHIV-infected rhesus macaques
- PMID: 28222180
- PMCID: PMC5319772
- DOI: 10.1371/journal.pone.0172524
Pre-existing neutralizing antibody mitigates B cell dysregulation and enhances the Env-specific antibody response in SHIV-infected rhesus macaques
Abstract
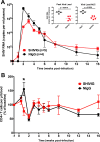
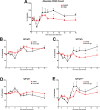
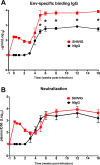
Our central hypothesis is that protection against HIV infection will be powerfully influenced by the magnitude and quality of the B cell response. Although sterilizing immunity, mediated by pre-formed abundant and potent antibodies is the ultimate goal for B cell-targeted HIV vaccine strategies, scenarios that fall short of this may still confer beneficial defenses against viremia and disease progression. We evaluated the impact of sub-sterilizing pre-existing neutralizing antibody on the B cell response to SHIV infection. Adult male rhesus macaques received passive transfer of a sub-sterilizing amount of polyclonal neutralizing immunoglobulin (Ig) purified from previously infected animals (SHIVIG) or control Ig prior to intra-rectal challenge with SHIVSF162P4 and extensive longitudinal sampling was performed. SHIVIG treated animals exhibited significantly reduced viral load and increased de novo Env-specific plasma antibody. Dysregulation of the B cell profile was grossly apparent soon after infection in untreated animals; exemplified by a ≈50% decrease in total B cells in the blood evident 2-3 weeks post-infection which was not apparent in SHIVIG treated animals. IgD+CD5+CD21+ B cells phenotypically similar to marginal zone-like B cells were highly sensitive to SHIV infection, becoming significantly decreased as early as 3 days post-infection in control animals, while being maintained in SHIVIG treated animals, and were highly correlated with the induction of Env-specific plasma antibody. These results suggest that B cell dysregulation during the early stages of infection likely contributes to suboptimal Env-specific B cell and antibody responses, and strategies that limit this dysregulation may enhance the host's ability to eliminate HIV.
Conflict of interest statement
Figures
Similar articles
-
Neutralizing polyclonal IgG present during acute infection prevents rapid disease onset in simian-human immunodeficiency virus SHIVSF162P3-infected infant rhesus macaques.J Virol. 2013 Oct;87(19):10447-59. doi: 10.1128/JVI.00049-13. Epub 2013 Jul 24. J Virol. 2013. PMID: 23885083 Free PMC article.
-
Rapid Development of gp120-Focused Neutralizing B Cell Responses during Acute Simian Immunodeficiency Virus Infection of African Green Monkeys.J Virol. 2015 Sep;89(18):9485-98. doi: 10.1128/JVI.01564-15. Epub 2015 Jul 8. J Virol. 2015. PMID: 26157116 Free PMC article.
-
Breadth and magnitude of antigen-specific antibody responses in the control of plasma viremia in simian immunodeficiency virus infected macaques.Virol J. 2016 Dec 1;13(1):200. doi: 10.1186/s12985-016-0652-x. Virol J. 2016. PMID: 27903274 Free PMC article.
-
Antibody-dependent cellular cytotoxicity in HIV infection.AIDS. 2018 Nov 13;32(17):2439-2451. doi: 10.1097/QAD.0000000000002011. AIDS. 2018. PMID: 30234611 Free PMC article. Review.
-
Dysregulation of humoral immunity in chronic infection.Immunol Cell Biol. 2020 Jul;98(6):456-466. doi: 10.1111/imcb.12338. Epub 2020 May 5. Immunol Cell Biol. 2020. PMID: 32275789 Review.
Cited by
-
Single-dose bNAb cocktail or abbreviated ART post-exposure regimens achieve tight SHIV control without adaptive immunity.Nat Commun. 2020 Jan 7;11(1):70. doi: 10.1038/s41467-019-13972-y. Nat Commun. 2020. PMID: 31911610 Free PMC article.
-
Passive and active antibody studies in primates to inform HIV vaccines.Expert Rev Vaccines. 2018 Feb;17(2):127-144. doi: 10.1080/14760584.2018.1425619. Epub 2018 Jan 19. Expert Rev Vaccines. 2018. PMID: 29307225 Free PMC article. Review.
References
-
- Montefiori DC, Karnasuta C, Huang Y, Ahmed H, Gilbert P, de Souza MS, et al. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. The Journal of infectious diseases. 2012;206(3):431–41. Epub 2012/05/29. PubMed Central PMCID: PMC3392187. 10.1093/infdis/jis367 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

