Longitudinal follow-up of adult survivors of Ewing sarcoma: A report from the Childhood Cancer Survivor Study
- PMID: 28222219
- PMCID: PMC5474122
- DOI: 10.1002/cncr.30627
Longitudinal follow-up of adult survivors of Ewing sarcoma: A report from the Childhood Cancer Survivor Study
Abstract
Background: Ewing sarcoma survivors (ESSs) are at increased risk for treatment-related complications. The incidence of treatment-related morbidity and late mortality with aging is unknown.
Methods: This study reports survival probabilities, estimated with the Kaplan-Meier method, and the cumulative incidence of cause-specific mortality and chronic conditions among ESSs in the Childhood Cancer Survivor Study who were treated between 1970 and 1986. Piecewise exponential models were used to estimate relative rates (RRs) and 95% confidence intervals (CIs) for these outcomes. Chronic conditions were graded with the Common Terminology Criteria for Adverse Events (version 4.03).
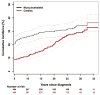
Results: Among 404 5-year ESSs (median age at last follow-up, 34.8 years; range, 9.1-54.8 years), the 35-year survival rate was 70% (95% CI, 66%-74%). Late recurrence (cumulative incidence at 35 years, 15.1%) was the most common cause of death, and it was followed by treatment-related causes (11.2%). There were 53 patients with subsequent neoplasms (SNs; cumulative incidence at 35 years, 24.0%), and 38 were malignant (14.3% at 35 years). The standardized incidence ratios were 377.1 (95% CI, 172.1-715.9) for osteosarcoma, 28.9 (95% CI, 3.2-104.2) for acute myeloid leukemia, 14.9 (95% CI, 7.9-25.5) for breast cancer, and 13.1 (95% CI, 4.8-28.5) for thyroid cancer. Rates of chronic conditions were highest for musculoskeletal (RR, 18.1; 95% CI, 12.8-25.7) and cardiac complications (RR, 1.8; 95% CI, 1.4-2.3). Thirty-five years after the diagnosis, the cumulative incidences of any chronic conditions and 2 or more chronic conditions were 84.6% (95% CI, 80.4%-88.8%) and 73.8% (95% CI, 67.8%-79.9%), respectively.
Conclusions: With extended follow-up, ESSs' risk for late mortality and SNs does not plateau. Treatment-related chronic conditions develop years after therapy, and this supports the need for lifelong follow-up. Cancer 2017;123:2551-60. © 2017 American Cancer Society.
Keywords: Ewing sarcoma; childhood cancer survivors; chronic health conditions; late mortality; treatment-related complications.
© 2017 American Cancer Society.
Conflict of interest statement
Figures
Comment in
-
Second malignancies in Ewing sarcoma survivors.Cancer. 2017 Oct 15;123(20):4075. doi: 10.1002/cncr.30922. Epub 2017 Aug 24. Cancer. 2017. PMID: 28837218 No abstract available.
-
Reply to Second malignancies in Ewing sarcoma survivors.Cancer. 2017 Oct 15;123(20):4075-4076. doi: 10.1002/cncr.30921. Epub 2017 Aug 24. Cancer. 2017. PMID: 28837220 Free PMC article. No abstract available.
References
-
- Jurgens H, Bier V, Dunst J, et al. [The German Society of Pediatric Oncology Cooperative Ewing Sarcoma Studies CESS 81/86: report after 6 1/2 years] Klin Padiatr. 1988;200:243–252. - PubMed
-
- Bacci G, Ferrari S, Bertoni F, et al. Prognostic factors in nonmetastatic Ewing's sarcoma of bone treated with adjuvant chemotherapy: analysis of 359 patients at the Istituto Ortopedico Rizzoli. J Clin Oncol. 2000;18:4–11. - PubMed
-
- Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348:694–701. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical