Restoring Cystic Fibrosis Transmembrane Conductance Regulator Function Reduces Airway Bacteria and Inflammation in People with Cystic Fibrosis and Chronic Lung Infections
- PMID: 28222269
- PMCID: PMC5476912
- DOI: 10.1164/rccm.201609-1954OC
Restoring Cystic Fibrosis Transmembrane Conductance Regulator Function Reduces Airway Bacteria and Inflammation in People with Cystic Fibrosis and Chronic Lung Infections
Abstract
Rationale: Previous work indicates that ivacaftor improves cystic fibrosis transmembrane conductance regulator (CFTR) activity and lung function in people with cystic fibrosis and G551D-CFTR mutations but does not reduce density of bacteria or markers of inflammation in the airway. These findings raise the possibility that infection and inflammation may progress independently of CFTR activity once cystic fibrosis lung disease is established.
Objectives: To better understand the relationship between CFTR activity, airway microbiology and inflammation, and lung function in subjects with cystic fibrosis and chronic airway infections.
Methods: We studied 12 subjects with G551D-CFTR mutations and chronic airway infections before and after ivacaftor. We measured lung function, sputum bacterial content, and inflammation, and obtained chest computed tomography scans.
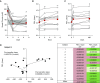
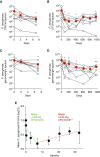
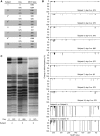
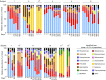
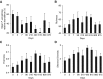
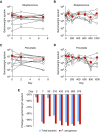
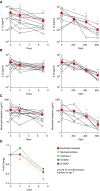
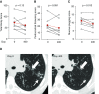
Measurements and main results: Ivacaftor produced rapid decreases in sputum Pseudomonas aeruginosa density that began within 48 hours and continued in the first year of treatment. However, no subject eradicated their infecting P. aeruginosa strain, and after the first year P. aeruginosa densities rebounded. Sputum total bacterial concentrations also decreased, but less than P. aeruginosa. Sputum inflammatory measures decreased significantly in the first week of treatment and continued to decline over 2 years. Computed tomography scans obtained before and 1 year after ivacaftor treatment revealed that ivacaftor decreased airway mucous plugging.
Conclusions: Ivacaftor caused marked reductions in sputum P. aeruginosa density and airway inflammation and produced modest improvements in radiographic lung disease in subjects with G551D-CFTR mutations. However, P. aeruginosa airway infection persisted. Thus, measures that control infection may be required to realize the full benefits of CFTR-targeting treatments.
Keywords: Pseudomonas aeruginosa; cystic fibrosis; inflammation; ivacaftor.
Figures
Comment in
-
Pseudomonas aeruginosa Infection after CFTR Restoration. One Step Back, One Step Forward.Am J Respir Crit Care Med. 2017 Jun 15;195(12):1550-1552. doi: 10.1164/rccm.201701-0220ED. Am J Respir Crit Care Med. 2017. PMID: 28617081 No abstract available.
References
-
- Welsh MJ, Ramsey BW, Accurso F, Cutting GR.Cystic fibrosis Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Vogelstein B.editorsThe metabolic and molecular basis of inherited diseases New York: McGraw-Hill; 20015121–5189.
-
- Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168:918–951. - PubMed
-
- Rowe SM, Heltshe SL, Gonska T, Donaldson SH, Borowitz D, Gelfond D, Sagel SD, Khan U, Mayer-Hamblett N, Van Dalfsen JM, et al. GOAL Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med. 2014;190:175–184. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

