VPS35, the Retromer Complex and Parkinson's Disease
- PMID: 28222538
- PMCID: PMC5438477
- DOI: 10.3233/JPD-161020
VPS35, the Retromer Complex and Parkinson's Disease
Abstract
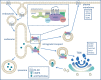
Mutations in the vacuolar protein sorting 35 ortholog (VPS35) gene encoding a core component of the retromer complex, have recently emerged as a new cause of late-onset, autosomal dominant familial Parkinson's disease (PD). A single missense mutation, AspD620Asn (D620N), has so far been unambiguously identified to cause PD in multiple individuals and families worldwide. The exact molecular mechanism(s) by which VPS35 mutations induce progressive neurodegeneration in PD are not yet known. Understanding these mechanisms, as well as the perturbed cellular pathways downstream of mutant VPS35, is important for the development of appropriate therapeutic strategies. In this review, we focus on the current knowledge surrounding VPS35 and its role in PD. We provide a critical discussion of the emerging data regarding the mechanisms underlying mutant VPS35-mediated neurodegeneration gleaned from genetic cell and animal models and highlight recent advances that may provide insight into the interplay between VPS35 and several other PD-linked gene products (i.e. α-synuclein, LRRK2 and parkin) in PD. Present data support a role for perturbed VPS35 and retromer function in the pathogenesis of PD.
Keywords: LRRK2; Parkinson’s disease (PD); VPS35; autophagy; endosomal sorting; lysosome; mitochondria; parkin; retromer; α-synuclein.
Figures
References
-
- Lang AE, & Lozano AM (1998) Parkinson’s disease. Second of two parts. N Engl J Med, 339, 1130–1143. - PubMed
-
- Lang AE, & Lozano AM (1998) Parkinson’s disease. First of two parts. N Engl J Med, 339, 1044–1053. - PubMed
-
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, & Nussbaum RL (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science, 276, 2045–2047. - PubMed
-
- Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, & Gasser T (2004) Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron, 44, 601–607. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

