C-terminal region of apoptin affects chicken anemia virus replication and virulence
- PMID: 28222746
- PMCID: PMC5320637
- DOI: 10.1186/s12985-017-0713-9
C-terminal region of apoptin affects chicken anemia virus replication and virulence
Abstract
Background: Chicken anemia virus (CAV) causes anemia and immune suppression, which are important diseases in the poultry industry. CAV VP3, also referred as 'apoptin', has been shown to selectively kill tumor cells, raising great hopes for its utilization as an anticancer therapy. The ability of apoptin to induce apoptosis is closely related to its nuclear localization. The C-terminal region of apoptin contains a bipartite nuclear localization signals (NLS), and a nuclear export signal (NES) is located between the arms of the NLS. Most previous studies have expressed apoptin of different lengths in vitro to understand the relationship between its localization and its induction of apoptosis.
Methods: In this study, we investigated the replication of CAV and its induction of apoptosis in vitro and in vivo with VP3-truncated infectious virus. Quantitative PCR was used to detect viral replication in MDCC-MSB1 cells, and the viral localization was observed by confocal microscopy. Flow cytometry was uesed to analyze virus-induced apoptosis in MDCC-MSB1 cells. Additionally, chickens infected with the rescued viruses compared with the parental virus rM9905 to evaluate the viral replication in vivo and virulence.
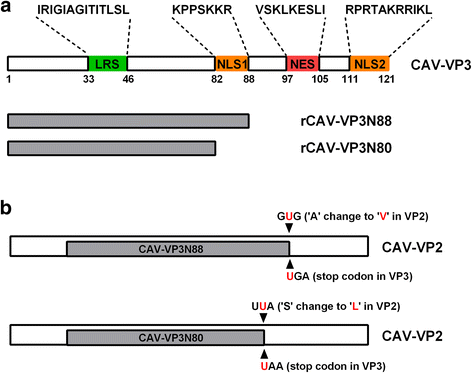
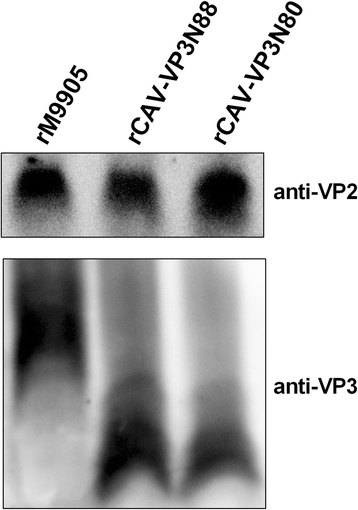
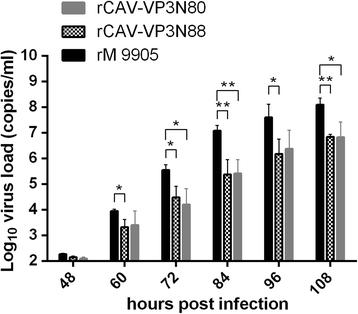
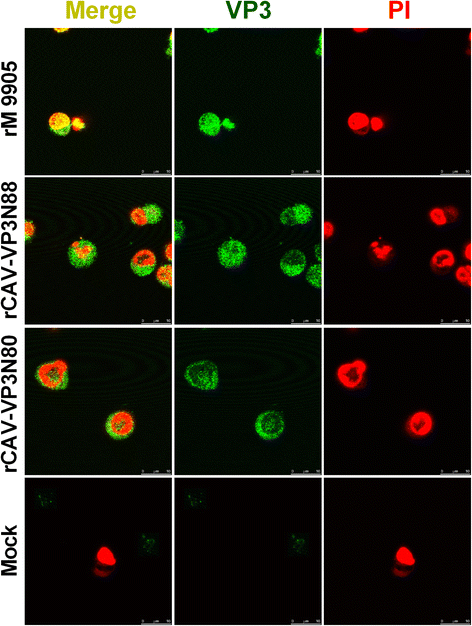
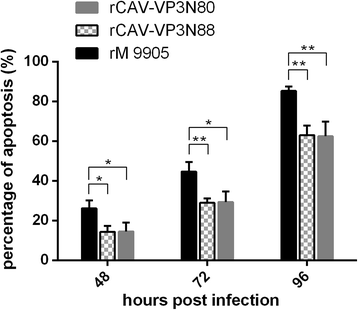
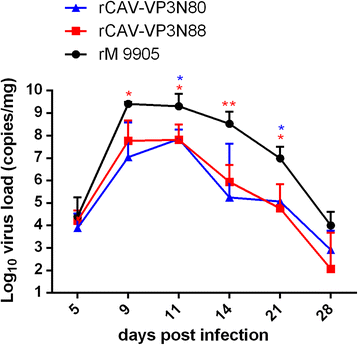
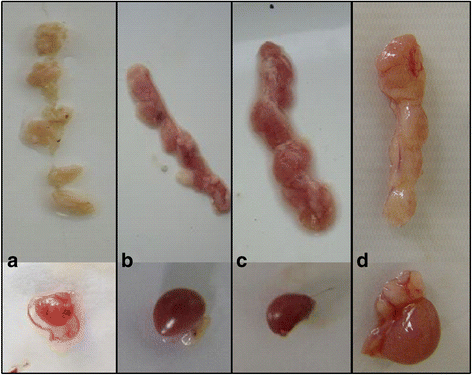
Results: Based on the infectious clone, we rescued two viruses in which were deleted NES-NLS2 (rCAV-VP3N88) or NLS1-NES-NLS2 (rCAV-VP3N80) in the C-terminal region of apoptin. The viral load of rCAV-VP3N88 decreased significantly between 60 and 108 hpi, and was always 10-100-fold lower than that of the parental virus rM9905. The levels of rCAV-VP3N80 were also 10-100-fold lower than that of rM9905 and declined significantly at three time points. There was almost no difference in the viral loads of rCAV-VP3N88 and rCAV-VP3N80. Additionally, rM9905 induced 85.39 ± 2.18% apoptosis at 96 hpi, whereas rCAV-VP3N88 and rCAV-VP3N80 induced 63.08 ± 4.78% and 62.56 ± 7.35% apoptosis, respectively, which were significantly (about 20%) lower than that induced by the parental virus. The rescued viruses altered the nuclear localization in MDCC-MSB1 cells. Moreover, deletion of C-terminal region of apoptin impaired viral replication in vivo and reduced the virulence of CAV in chickens.
Conclusions: In summary, we have demonstrated that the C-terminal deletion of apoptin in infectious CAV affected the replication of the virus. The deletion of the C-terminal region of apoptin not only significantly reduced viral replication in vitro but also reduced its induction of apoptosis, which correlated with the loss of its nuclear localization. The deletion of the C-terminal region of apoptin also impaired the replication of CAV and attenuated its virulence in chickens.
Keywords: Apoptin; Apoptosis; Chicken anemia virus; Replication; Virulence.
Figures

Similar articles
-
Chicken anemia virus induced apoptosis: underlying molecular mechanisms.Vet Microbiol. 2004 Feb 4;98(2):89-94. doi: 10.1016/j.vetmic.2003.10.003. Vet Microbiol. 2004. PMID: 14741120 Review.
-
VP2 of Chicken Anaemia Virus Interacts with Apoptin for Down-regulation of Apoptosis through De-phosphorylated Threonine 108 on Apoptin.Sci Rep. 2017 Nov 1;7(1):14799. doi: 10.1038/s41598-017-14558-8. Sci Rep. 2017. PMID: 29093508 Free PMC article.
-
Replication of chicken anemia virus (CAV) requires apoptin and is complemented by VP3 of human torque teno virus (TTV).Virology. 2009 Mar 1;385(1):85-92. doi: 10.1016/j.virol.2008.10.043. Epub 2008 Dec 16. Virology. 2009. PMID: 19091368
-
Apoptin nucleocytoplasmic shuttling is required for cell type-specific localization, apoptosis, and recruitment of the anaphase-promoting complex/cyclosome to PML bodies.J Virol. 2006 Aug;80(15):7535-45. doi: 10.1128/JVI.02741-05. J Virol. 2006. PMID: 16840333 Free PMC article.
-
Apoptin, a tumor-selective killer.Biochim Biophys Acta. 2009 Aug;1793(8):1335-42. doi: 10.1016/j.bbamcr.2009.04.002. Epub 2009 Apr 15. Biochim Biophys Acta. 2009. PMID: 19374922 Review.
Cited by
-
Genotype F of Echovirus 25 with multiple recombination pattern have been persistently and extensively circulating in Chinese mainland.Sci Rep. 2024 Feb 8;14(1):3212. doi: 10.1038/s41598-024-53513-2. Sci Rep. 2024. PMID: 38332009 Free PMC article.
-
Porcine circovirus type 2 ORF3 protein induces apoptosis in melanoma cells.BMC Cancer. 2018 Dec 10;18(1):1237. doi: 10.1186/s12885-018-5090-2. BMC Cancer. 2018. PMID: 30526524 Free PMC article.
-
Isolation and genomic characterization of chicken infectious anemia virus in Jiangsu province of China during 2020-2022.Front Vet Sci. 2024 Mar 14;11:1378120. doi: 10.3389/fvets.2024.1378120. eCollection 2024. Front Vet Sci. 2024. PMID: 38550786 Free PMC article.
-
Different Dynamic Distribution in Chickens and Ducks of the Hypervirulent, Novel Genotype Fowl Adenovirus Serotype 4 Recently Emerged in China.Front Microbiol. 2017 Jun 6;8:1005. doi: 10.3389/fmicb.2017.01005. eCollection 2017. Front Microbiol. 2017. PMID: 28634474 Free PMC article.
-
Retrospective Investigation and Genetic Variation Analysis of Chicken Infectious Anemia in Shandong Province, 2020-2022.Vet Sci. 2023 Mar 29;10(4):263. doi: 10.3390/vetsci10040263. Vet Sci. 2023. PMID: 37104419 Free PMC article.
References
-
- DanenVan Oorschot AA, Zhang YH, Leliveld SR, Rohn JL, Seelen MC, Bolk MW, Van Zon A, Erkeland SJ, Abrahams JP, Mumberg D, Noteborn MH. Importance of nuclear localization of apoptin for tumor-specific induction of apoptosis. J Biol Chem. 2003;278:27729–27736. doi: 10.1074/jbc.M303114200. - DOI - PubMed
-
- DanenVan Oorschot AA, Fischer DF, Grimbergen JM, Klein B, Zhuang S, Falkenburg JH, Backendorf C, Quax PH, Van Der Eb AJ, Noteborn MH. Apoptin induces apoptosis in human transformed and malignant cells but not in normal cells. Proc Natl Acad Sci U S A. 1997;94:5843–5847. doi: 10.1073/pnas.94.11.5843. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

