Knockdown of SLC41A1 magnesium transporter promotes mineralization and attenuates magnesium inhibition during osteogenesis of mesenchymal stromal cells
- PMID: 28222767
- PMCID: PMC5320718
- DOI: 10.1186/s13287-017-0497-2
Knockdown of SLC41A1 magnesium transporter promotes mineralization and attenuates magnesium inhibition during osteogenesis of mesenchymal stromal cells
Abstract
Background: Magnesium is essential for numerous physiological functions. Magnesium exists mostly in bone and the amount is dynamically regulated by skeletal remodeling. Accelerating bone mass loss occurs when magnesium intake is insufficient; whereas high magnesium could lead to mineralization defects. However, the underlying magnesium regulatory mechanisms remain elusive. In the present study, we investigated the effects of high extracellular magnesium concentration on osteogenic differentiation of mesenchymal stromal/stem cells (MSCs) and the role of magnesium transporter SLC41A1 in the mineralization process.
Methods: Murine MSCs derived from the bone marrow of BALB/c mouse or commercially purchased human MSCs were treated with osteogenic induction medium containing 5.8 mM magnesium chloride and the osteogenic differentiation efficiency was compared with that of MSCs in normal differentiation medium containing 0.8 mM magnesium chloride by cell morphology, gene expression profile of osteogenic markers, and Alizarin Red staining. Slc41a1 gene knockdown in MSCs was performed by siRNA transfection using Lipofectamine RNAiMAX, and the differentiation efficiency of siRNA-treated MSCs was also assessed.
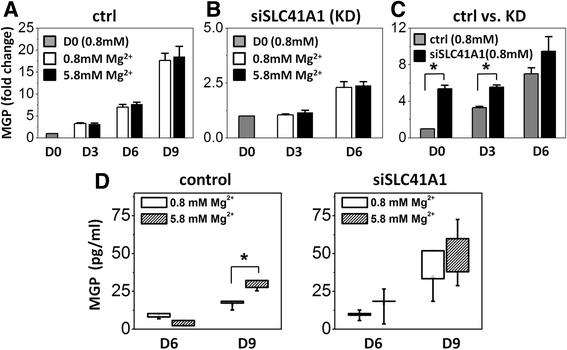
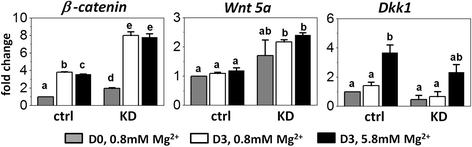
Results: High concentration of extracellular magnesium ion inhibited mineralization during osteogenic differentiation of MSCs. Early osteogenic marker genes including osterix, alkaline phosphatase, and type I collagen were significantly downregulated in MSCs under high concentration of magnesium, whereas late marker genes such as osteopontin, osteocalcin, and bone morphogenetic protein 2 were upregulated with statistical significance compared with those in normal differentiation medium containing 0.8 mM magnesium. siRNA treatment targeting SLC41A1 magnesium transporter, a member of the solute carrier family with a predominant Mg2+ efflux system, accelerated the mineralization process and ameliorated the inhibition of mineralization caused by high concentration of magnesium. High concentration of magnesium significantly upregulated Dkk1 gene expression and the upregulation was attenuated after the Slc41a1 gene was knocked down. Immunofluorescent staining showed that Slc41a1 gene knockdown promoted the translocation of phosphorylated β-catenin into nuclei. In addition, secreted MGP protein was elevated after Slc41a1 was knocked down.
Conclusions: High concentration of extracellular magnesium modulates gene expression of MSCs during osteogenic differentiation and inhibits the mineralization process. Additionally, we identified magnesium transporter SLC41A1 that regulates the interaction of magnesium and MSCs during osteogenic differentiation. Wnt signaling is suggested to be involved in SLC41A1-mediated regulation. Tissue-specific SLC41A1 could be a potential treatment for bone mass loss; in addition, caution should be taken regarding the role of magnesium in osteoporosis and the design of magnesium alloys for implantation.
Keywords: Magnesium transporter; Mesenchymal stromal cells; Mineralization; Osteogenic differentiation; SLC41A1.
Figures




Similar articles
-
Berberine promotes bone marrow-derived mesenchymal stem cells osteogenic differentiation via canonical Wnt/β-catenin signaling pathway.Toxicol Lett. 2016 Jan 5;240(1):68-80. doi: 10.1016/j.toxlet.2015.10.007. Epub 2015 Oct 22. Toxicol Lett. 2016. PMID: 26478571
-
Osteocalcin Mediates Biomineralization during Osteogenic Maturation in Human Mesenchymal Stromal Cells.Int J Mol Sci. 2017 Jan 17;18(1):159. doi: 10.3390/ijms18010159. Int J Mol Sci. 2017. PMID: 28106724 Free PMC article.
-
Synergistic interaction of platelet derived growth factor (PDGF) with the surface of PLLA/Col/HA and PLLA/HA scaffolds produces rapid osteogenic differentiation.Colloids Surf B Biointerfaces. 2016 Mar 1;139:68-78. doi: 10.1016/j.colsurfb.2015.11.053. Epub 2015 Nov 28. Colloids Surf B Biointerfaces. 2016. PMID: 26700235
-
[Regulation of osteogenic differentiation of mesenchimal stem sells of bone marrow].Ross Fiziol Zh Im I M Sechenova. 2013 Apr;99(4):417-32. Ross Fiziol Zh Im I M Sechenova. 2013. PMID: 23862383 Review. Russian.
-
[RESEARCH PROGRESS OF Hedgehog SIGNALING PATHWAY IN REGULATING BONE FORMATION AND OSTEOGENIC DIFFERENTIATION OF BONE MESENCHYMAL STEM CELLS].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2016 Dec 8;30(12):1545-1550. doi: 10.7507/1002-1892.20160318. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2016. PMID: 29786349 Review. Chinese.
Cited by
-
The Impact of Trace Elements on Osteoarthritis.Front Med (Lausanne). 2021 Dec 23;8:771297. doi: 10.3389/fmed.2021.771297. eCollection 2021. Front Med (Lausanne). 2021. PMID: 35004740 Free PMC article. Review.
-
A Degradable and Osteogenic Mg-Based MAO-MT-PLGA Drug/Ion Delivery System for Treating an Osteoporotic Fracture.Pharmaceutics. 2022 Jul 16;14(7):1481. doi: 10.3390/pharmaceutics14071481. Pharmaceutics. 2022. PMID: 35890376 Free PMC article.
-
Surface Modification of Biodegradable Mg-Based Scaffolds for Human Mesenchymal Stem Cell Proliferation and Osteogenic Differentiation.Materials (Basel). 2021 Jan 18;14(2):441. doi: 10.3390/ma14020441. Materials (Basel). 2021. PMID: 33477485 Free PMC article.
-
Analysis of the CPZ/Wnt4 osteogenic pathway for high-bonding-strength composite-coated magnesium scaffolds through transcriptomics.Mater Today Bio. 2024 Sep 8;28:101234. doi: 10.1016/j.mtbio.2024.101234. eCollection 2024 Oct. Mater Today Bio. 2024. PMID: 39309165 Free PMC article.
-
Magnesium Deprivation Potentiates Human Mesenchymal Stem Cell Transcriptional Remodeling.Int J Mol Sci. 2018 May 9;19(5):1410. doi: 10.3390/ijms19051410. Int J Mol Sci. 2018. PMID: 29747379 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

