Advanced biosensors for detection of pathogens related to livestock and poultry
- PMID: 28222780
- PMCID: PMC5320782
- DOI: 10.1186/s13567-017-0418-5
Advanced biosensors for detection of pathogens related to livestock and poultry
Abstract
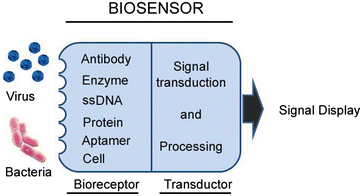
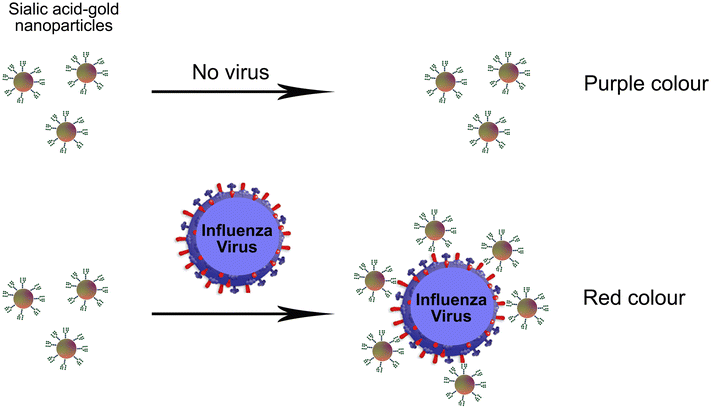
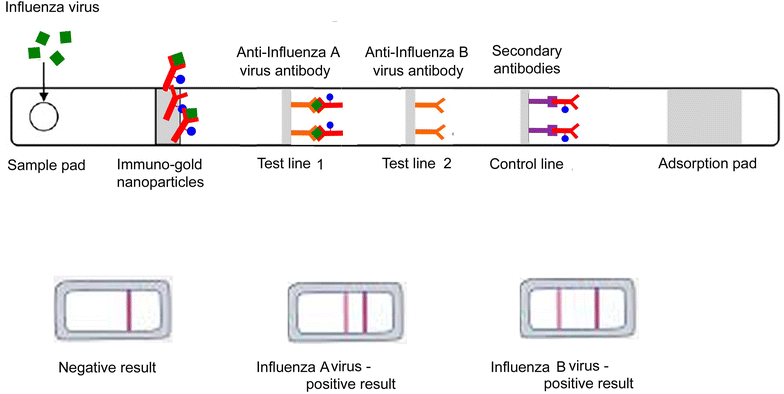
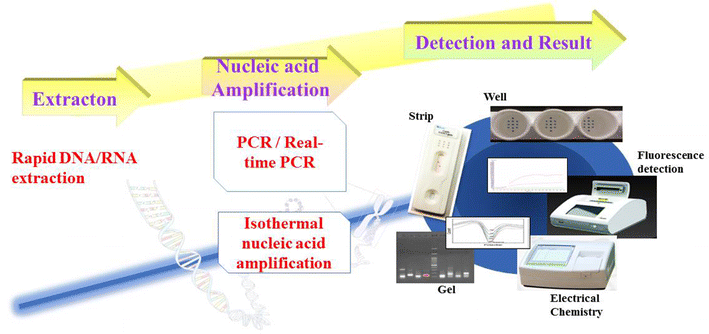
Infectious animal diseases caused by pathogenic microorganisms such as bacteria and viruses threaten the health and well-being of wildlife, livestock, and human populations, limit productivity and increase significantly economic losses to each sector. The pathogen detection is an important step for the diagnostics, successful treatment of animal infection diseases and control management in farms and field conditions. Current techniques employed to diagnose pathogens in livestock and poultry include classical plate-based methods and conventional biochemical methods as enzyme-linked immunosorbent assays (ELISA). These methods are time-consuming and frequently incapable to distinguish between low and highly pathogenic strains. Molecular techniques such as polymerase chain reaction (PCR) and real time PCR (RT-PCR) have also been proposed to be used to diagnose and identify relevant infectious disease in animals. However these DNA-based methodologies need isolated genetic materials and sophisticated instruments, being not suitable for in field analysis. Consequently, there is strong interest for developing new swift point-of-care biosensing systems for early detection of animal diseases with high sensitivity and specificity. In this review, we provide an overview of the innovative biosensing systems that can be applied for livestock pathogen detection. Different sensing strategies based on DNA receptors, glycan, aptamers and antibodies are presented. Besides devices still at development level some are validated according to standards of the World Organization for Animal Health and are commercially available. Especially, paper-based platforms proposed as an affordable, rapid and easy to perform sensing systems for implementation in field condition are included in this review.
Figures
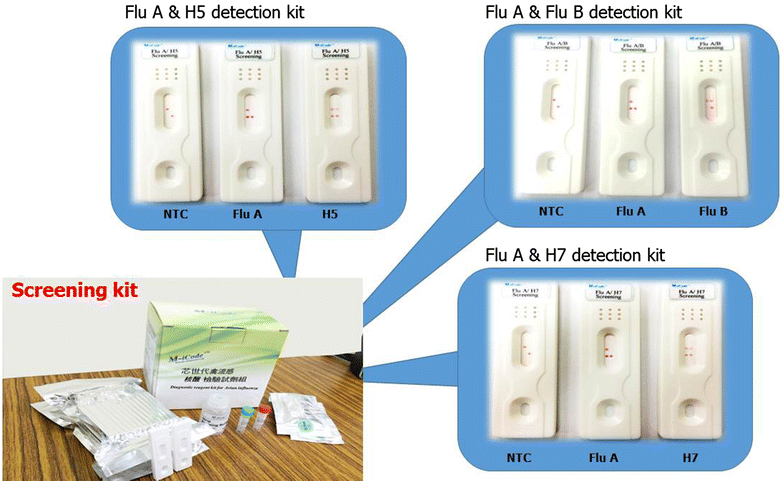
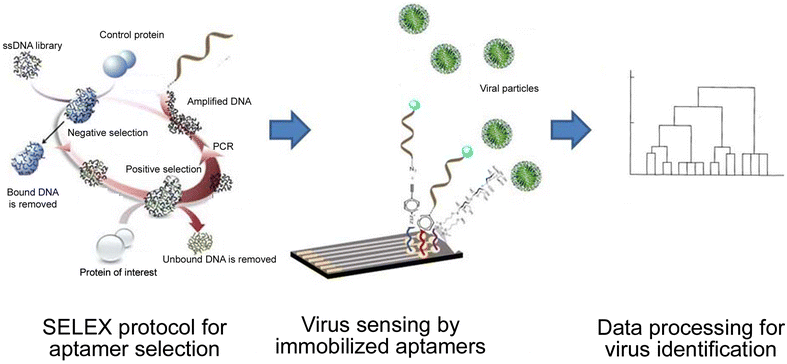
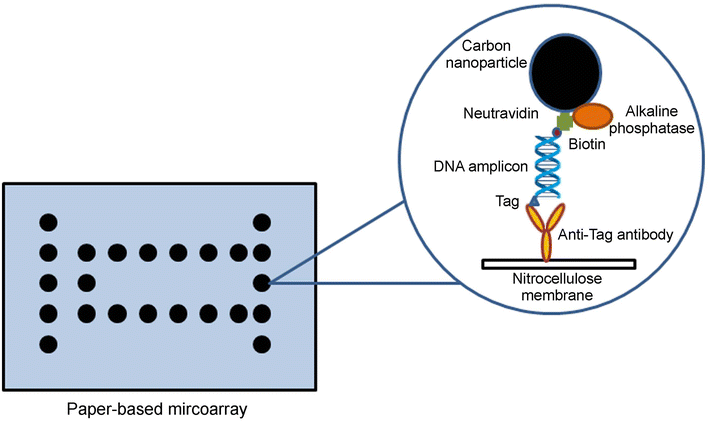
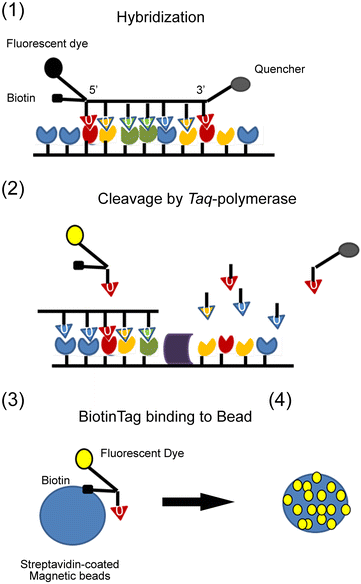

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

