Modified mRNA Vaccines Protect against Zika Virus Infection
- PMID: 28222903
- PMCID: PMC5388441
- DOI: 10.1016/j.cell.2017.02.017
Modified mRNA Vaccines Protect against Zika Virus Infection
Erratum in
-
Modified mRNA Vaccines Protect against Zika Virus Infection.Cell. 2017 Mar 23;169(1):176. doi: 10.1016/j.cell.2017.03.016. Cell. 2017. PMID: 28340344 No abstract available.
Abstract
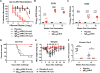
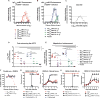
The emergence of ZIKV infection has prompted a global effort to develop safe and effective vaccines. We engineered a lipid nanoparticle (LNP) encapsulated modified mRNA vaccine encoding wild-type or variant ZIKV structural genes and tested immunogenicity and protection in mice. Two doses of modified mRNA LNPs encoding prM-E genes that produced virus-like particles resulted in high neutralizing antibody titers (∼1/100,000) that protected against ZIKV infection and conferred sterilizing immunity. To offset a theoretical concern of ZIKV vaccines inducing antibodies that cross-react with the related dengue virus (DENV), we designed modified prM-E RNA encoding mutations destroying the conserved fusion-loop epitope in the E protein. This variant protected against ZIKV and diminished production of antibodies enhancing DENV infection in cells or mice. A modified mRNA vaccine can prevent ZIKV disease and be adapted to reduce the risk of sensitizing individuals to subsequent exposure to DENV, should this become a clinically relevant concern.
Keywords: Dengue virus; RNA vaccine; Zika virus; antibody neutralization; flavivirus; immunity; pathogenesis; protection.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
M.S.D. is a consultant for Inbios, Visterra, and Takeda Pharmaceuticals and on the Scientific Advisory Boards of Moderna and OvaGene. S.H., S.B., and G.C. are employees of Valera LLC, a Moderna Venture focusing on the development of therapeutic approaches for Infectious Diseases, including ZIKV mRNA vaccines.
Figures


Comment in
-
An mRNA-based vaccine strategy against Zika.Cell Res. 2017 Sep;27(9):1077-1078. doi: 10.1038/cr.2017.53. Epub 2017 Apr 11. Cell Res. 2017. PMID: 28397799 Free PMC article.
-
Zika Virus Vaccines - A Full Field and Looking for the Closers.N Engl J Med. 2017 May 11;376(19):1883-1886. doi: 10.1056/NEJMcibr1701402. N Engl J Med. 2017. PMID: 28490001 No abstract available.
References
-
- Barba-Spaeth G, Dejnirattisai W, Rouvinski A, Vaney MC, Medits I, Sharma A, Simon-Loriere E, Sakuntabhai A, Cao-Lormeau VM, Haouz A, et al. Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature. 2016;536:48–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

